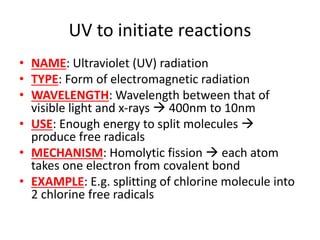
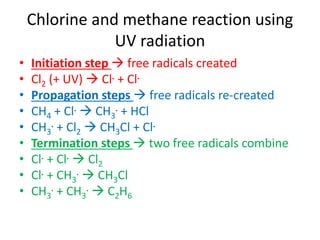
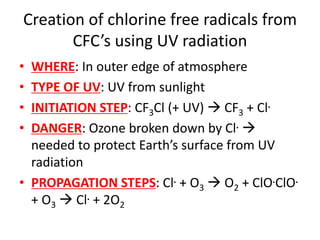
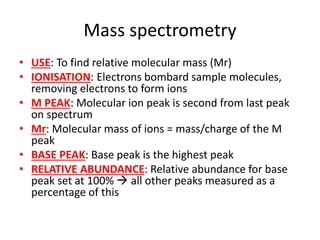
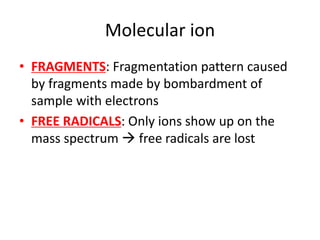
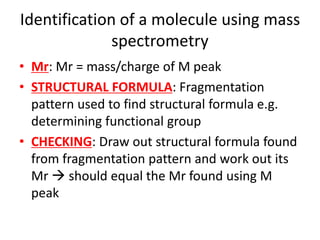
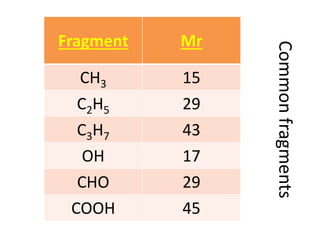
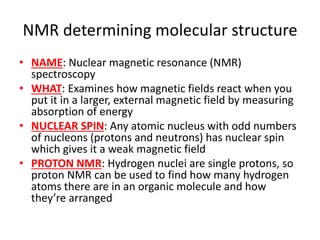
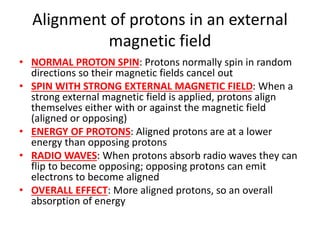
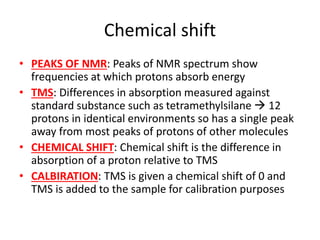
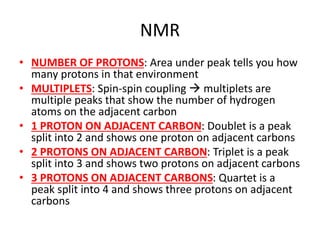
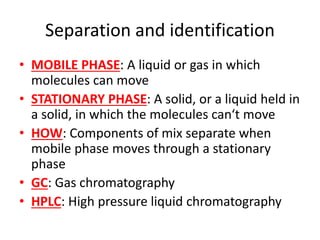
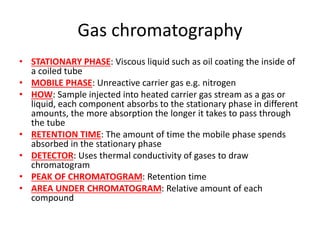
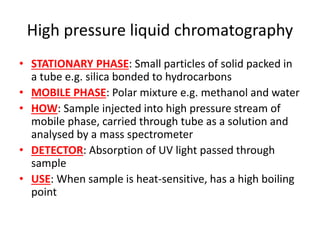
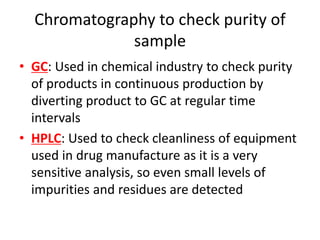
Ultraviolet (UV) radiation and microwaves can be used to initiate organic reactions. UV radiation provides enough energy to homolytically cleave bonds and generate free radicals to propagate reactions. Microwaves are used for heating through interactions with polar molecules. Mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, chromatography, and chemical tests are techniques used to analyze organic compounds and determine molecular structure.













![Oxidation of aldehydes to carboxylic
acids
• REAGENT: Potassium dichromate (6)
• CONDITIONS: Heated under reflux with dil
sulfuric acid
• POSITIVE RESULT: Orange green
• EQUATION: RCH=O + [O] RC=OOH](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-35-320.jpg)
![Reduction of carbonyls
• ALDEHYDES: Form primary alcohols when
reduced
• EQUATION: RCH=O + 2[H] RCH2OH
• KETONES: Form secondary alcohols when
reduced
• EQUATION: RCR’=O + 2[H] RCHR’OH
• REAGENT: LiALH4 (lithium aluminium hydride)
• CONDITIONS: In dry ether](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-36-320.jpg)




![Formation of carboxylic acids
OXIDATION OF PRIMARY
ALCOHOLS AND ALDEHYDES
• OVERVIEW: Primary alcohol
Aldehyde Carboxylic
acid
• EQUATION: RCH2OH + [O]
RCH=O + [O] RC=OOH
HYDROLYSIS OF NITRILES
• CONDITIONS: Heat under
reflux with dilute
hydrochloric acid, distil off
carboxylic acid
• EQUATION: CH3CN + 2H2O +
HCl CH3C=OOH](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-41-320.jpg)
![Reactions
• TYPE: Reduction
• REAGENT: LiAlH4
• CONDITIONS: Dry ether
• PRODUCT: Primary
alcohol
• REAGENT: PCl5
[phosphorus (5)
chloride]
• PRODUCT: Acyl chloride,
POCl3, HCl](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-43-320.jpg)





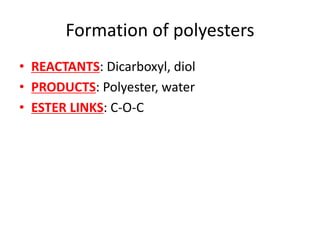

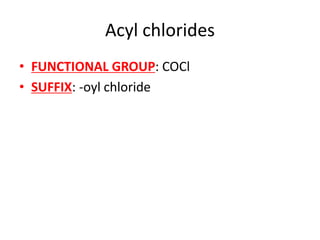
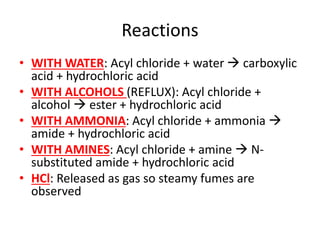

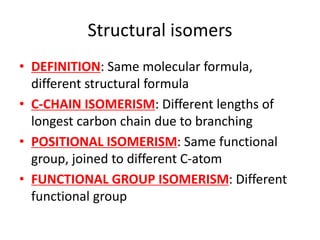
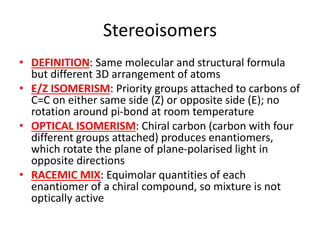
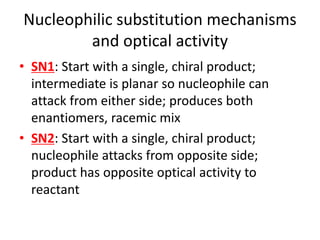

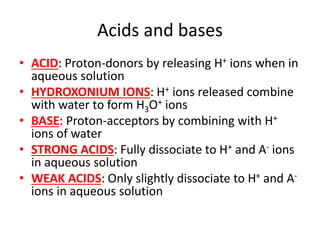
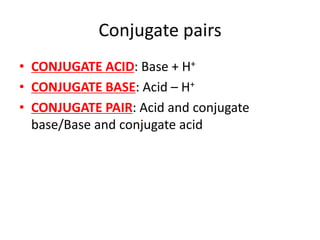
![Water
• AS ACID: Donates a proton to form hydroxide
ions
• AS BASE: Accepts a proton to form hydroxonium
ions
• DISSOCIATION: Very little dissociation;
equilibrium lies on left
• IONIC PRODUCT OF WATER: Kw = [H+][OH-]
• VALUE OF Kw: At 298K = 1.0x10-14 mol2dm-6
• pKw = -logKw
• VALUE OF pKw: At 298K = 14](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-62-320.jpg)
![Acidity of solutions
• NEUTRAL: [H+] = [OH-]
• ACIDIC: [H+] > [OH-]
• ALKALINE: [H+] < [OH-]](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-63-320.jpg)

![pH
• pH = -log[H+]
• STRONG ACIDS: [H+] = [HA]
• pOH = 14 – pH
• Ka = [H+][A-] / [HA]
• WEAK ACID: [H+]2 = Ka[HA]
• ASSUMPTIONS FOR CALCULATING pH OF WEAK
ACID: [HA]initial = [HA]equilibrium; all H+ ions
come from acid (none from water)
• pKa = -logKa
• Ka = 10-pKa](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-65-320.jpg)


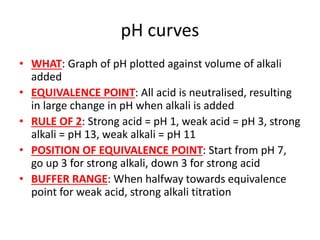

![Titration curves to find pKa of a weak
acid
• HALF-EQUIVALENCE POINT: pH = pKa
• Ka = 10-pKa
• [HA]2 = 10-pKa[HA]](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-70-320.jpg)

![Calculating pH
• Ka = [H+][OH-] / [HA]
• [H+] = Ka ([HA]/[OH-])
• pH = -log[H+]
• ASSUMPTIONS: [HA]initial = [HA]equilibrium;
[salt] = [OH-]](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-74-320.jpg)




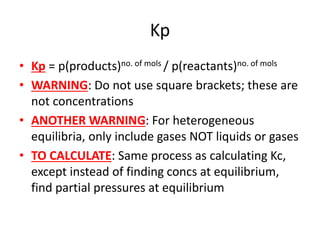
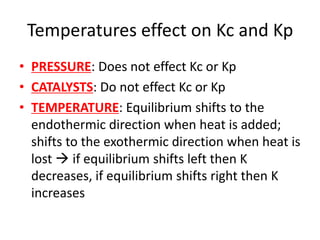
![Kc
• Kc = [products]no. of mols / [reactants]no. of mols
• TYPE OF EQUILIBRIUM: Only applies for
homogeneous equilibrium
• HETEROGENEOUS EQUILIBRIUM: If
heterogeneous mix of solids and gases or
solids and liquids then leave out the conc of
the solid
• WARNING: Do not use for mix of gases and
liquids](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-79-320.jpg)



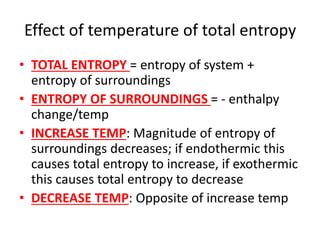

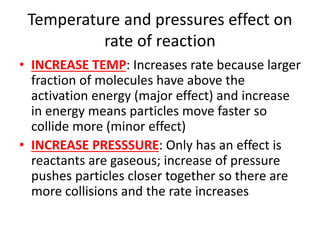




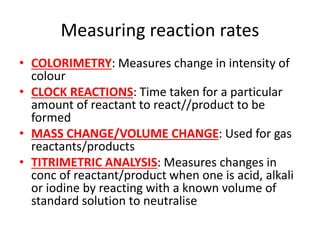
![Rate equations
• AVERAGE RATE = change in conc/change time
• RATE = k[A]x[B]y
• K = rate constant, constant at a particular
temp
• [A] AND [B] = concs of substances A and B
• X AND Y = partial orders
• OVERALL ORDER = x + y](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-106-320.jpg)

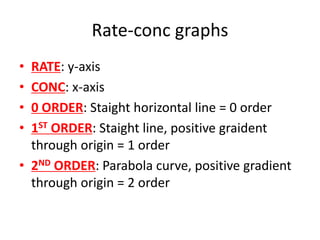
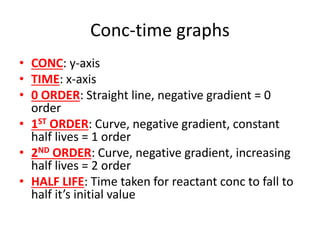

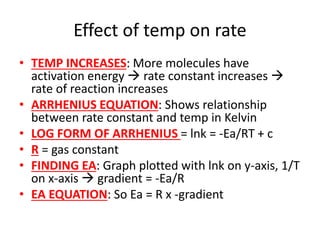
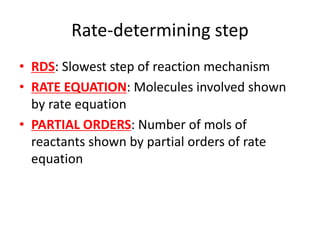
![Nucleophilic substitution SN1
• TYPE OF RX:Tertiary halogenoalkanes
• OVERALL ORDER: First order rate equation
• RDS: C-X bond breaking is rate determining
step
• FAST STEP: C+ being attacked by OH- is fast
step
• RATE = k[RX]](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-113-320.jpg)
![Nucleophilic substitution SN2
• TYPE OF RX: Primary halogenalkanes
• OVERALL ORDER: Second order rate equation
• RDS: Single step only rate-determining step
• RATE = k[RX][OH-]](https://image.slidesharecdn.com/chemistryedexcelunit4-230820030438-0d7f9966/85/Chemistry-Edexcel-Unit-4-pptx-114-320.jpg)

