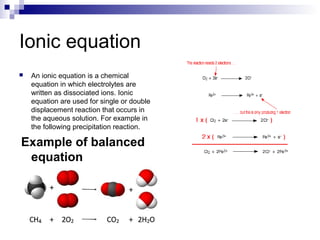
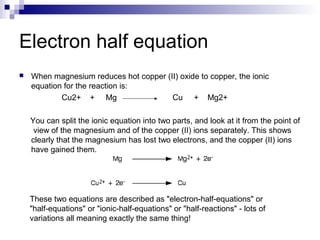
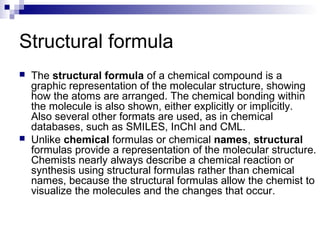
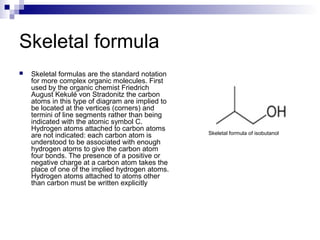
Chemical formulas and equations are used to represent chemical reactions and compounds. A chemical formula uses symbols to show the proportions of atoms in a compound without specifying structure. Empirical formulas show only atomic ratios while molecular formulas show exact atom counts. Structural formulas depict molecular structure and bonding. Skeletal formulas simplify complex organic structures by implying carbon and hydrogen atoms. Empirical formulas give the simplest whole number ratio of elements in a compound without isotopes or structure. Chemical equations balance reactants and products to represent chemical reactions, while ionic and half equations break reactions into component ion exchanges.


![Chemical equation
A chemical equation is the symbolic representation of a
chemical reaction where the reactant entities are given
on the left hand side and the product entities on the
right hand side.[1] The coefficients next to the symbols
and formulae of entities are the absolute values of the
stoichiometric numbers. The first chemical equation was
diagrammed by Jean Beguin in 1615.
A chemical equation consists of the chemical formulas of
the reactants (the starting substances) and the chemical
formula of the products (substances formed in the
chemical reaction).
The two are separated by an arrow symbol (, usually
read as "yields") and each
individual substance's chemical formula is separated
from
others by a plus sign.](https://image.slidesharecdn.com/chemicalformulaeandequations-130927044548-phpapp02/85/Chemical-formulae-and-equations-6-320.jpg)