Embed presentation
Download to read offline

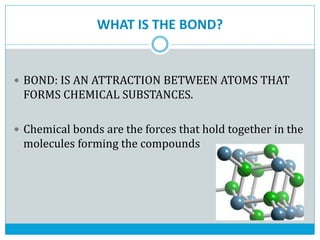
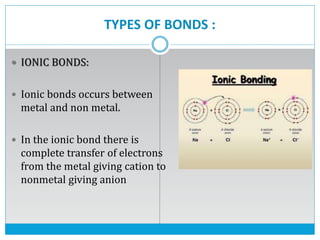
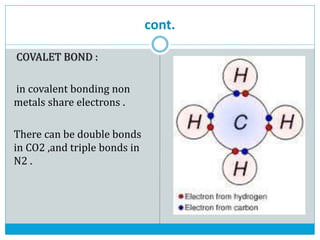
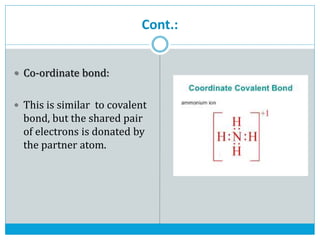
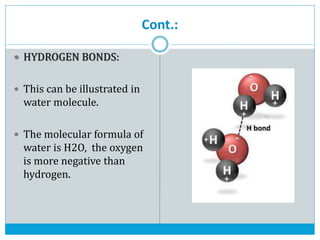


This document discusses the different types of chemical bonds: ionic bonds form between metals and nonmetals through the transfer of electrons from the metal to the nonmetal; covalent bonds involve the sharing of electron pairs between nonmetals to form molecules like CO2 and N2; coordinate bonds are similar to covalent bonds but involve one atom donating an electron pair; and hydrogen bonds occur when hydrogen atoms are attracted to slightly negative atoms like oxygen, as seen in water molecules. The document provides examples and brief explanations of each bond type.






