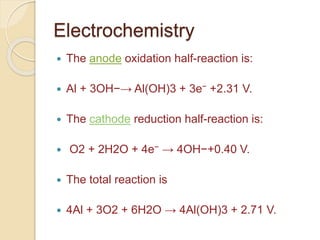
The document discusses the chem-e-car project, which utilizes aluminum-air (Al-Air) batteries to reduce vehicle pollution by converting chemical energy into mechanical energy. These batteries generate electricity through the reaction between atmospheric oxygen and aluminum, providing comparable efficiency and driving performance to gasoline vehicles. While Al-Air batteries are non-rechargeable, they can be mechanically replenished with recycled aluminum, highlighting their potential for various applications beyond automotive use.