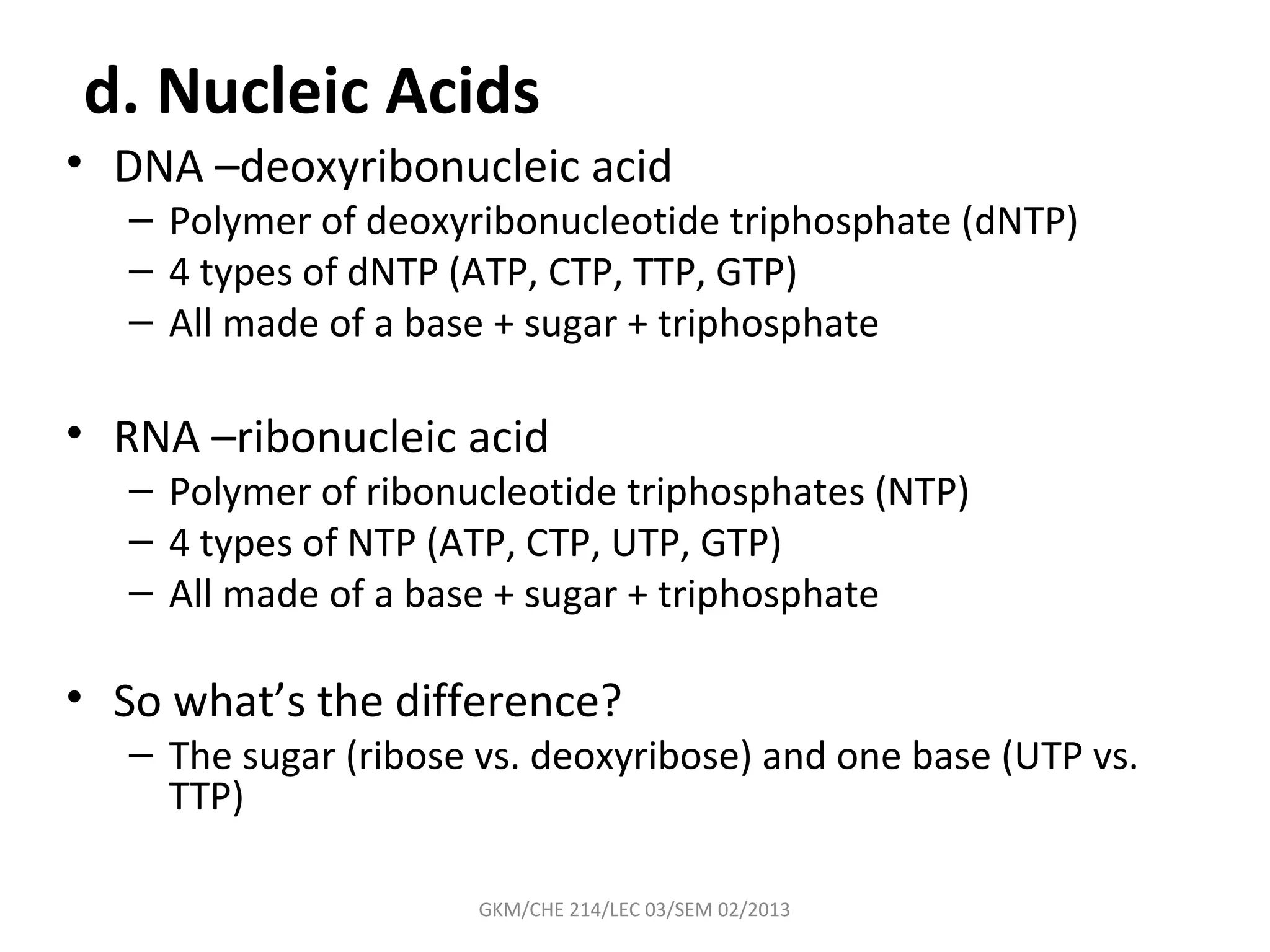
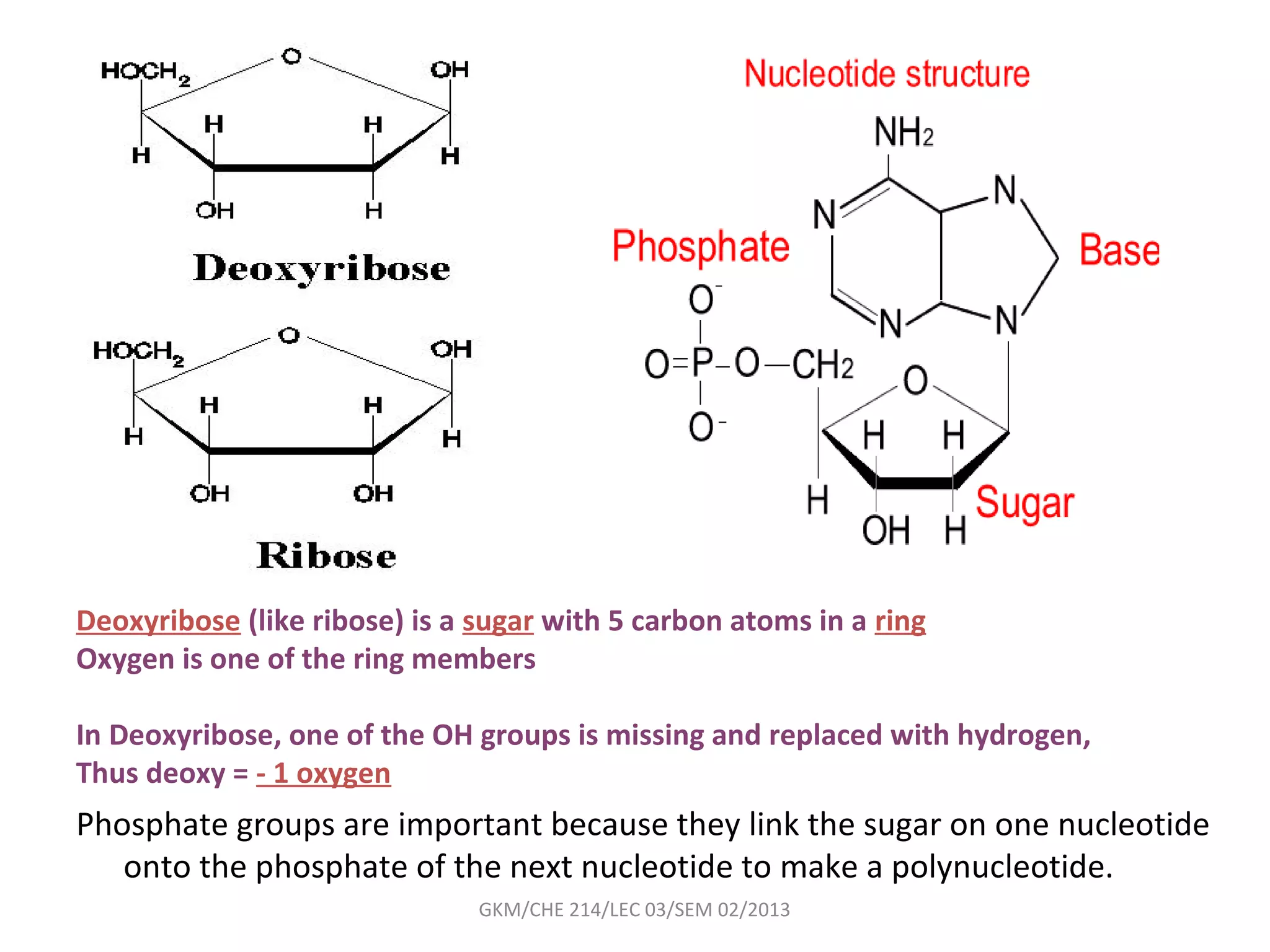
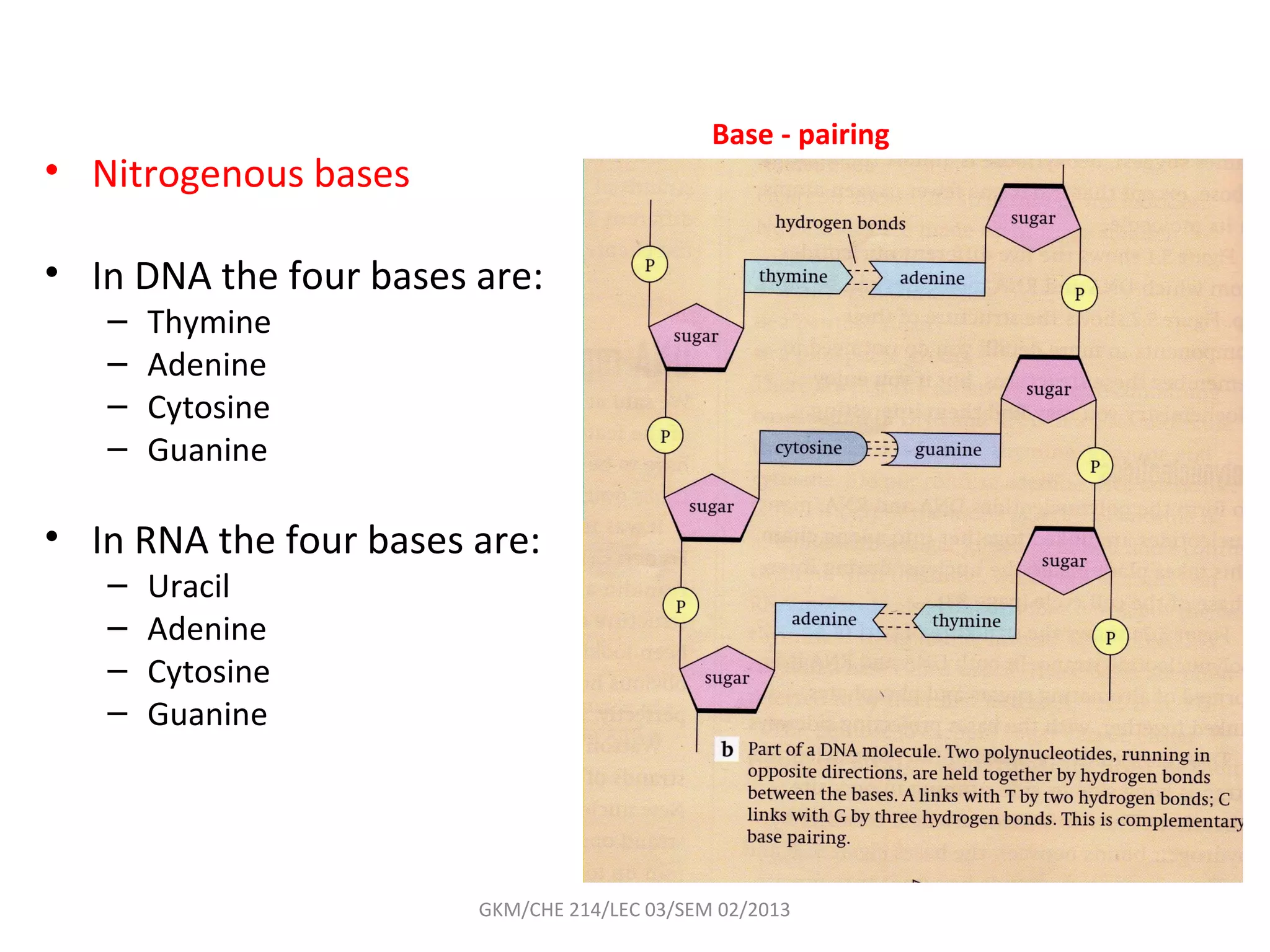
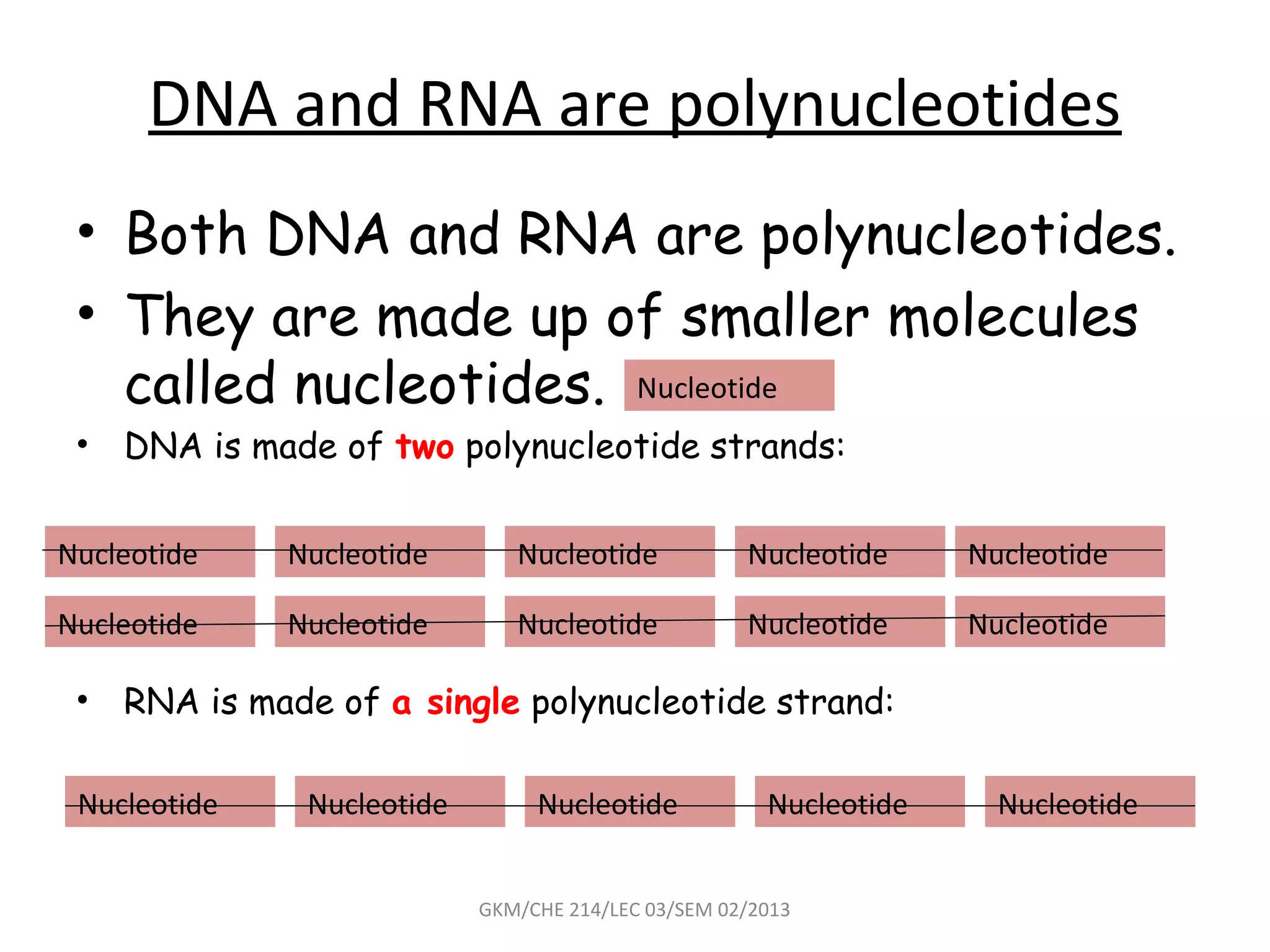
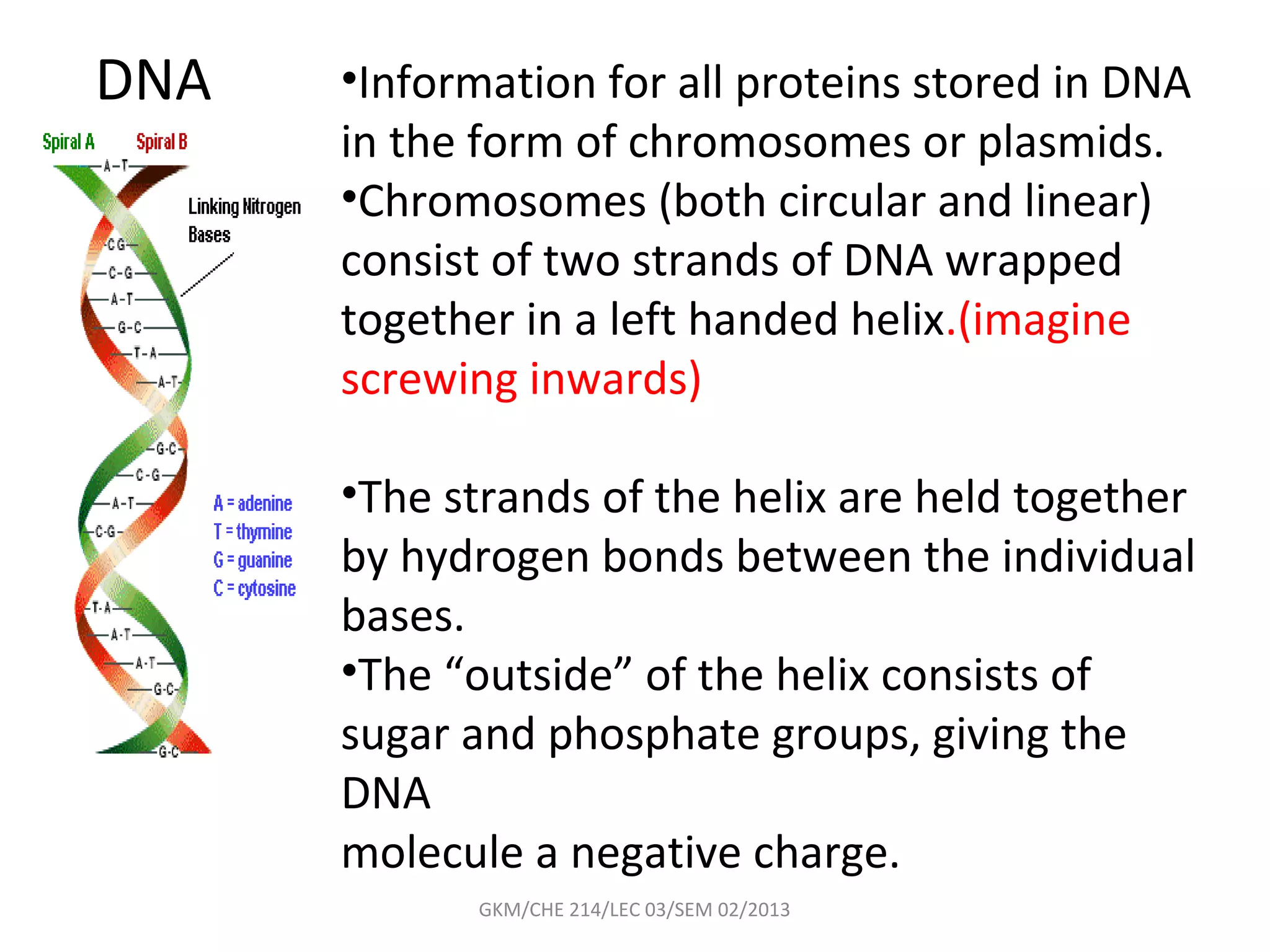
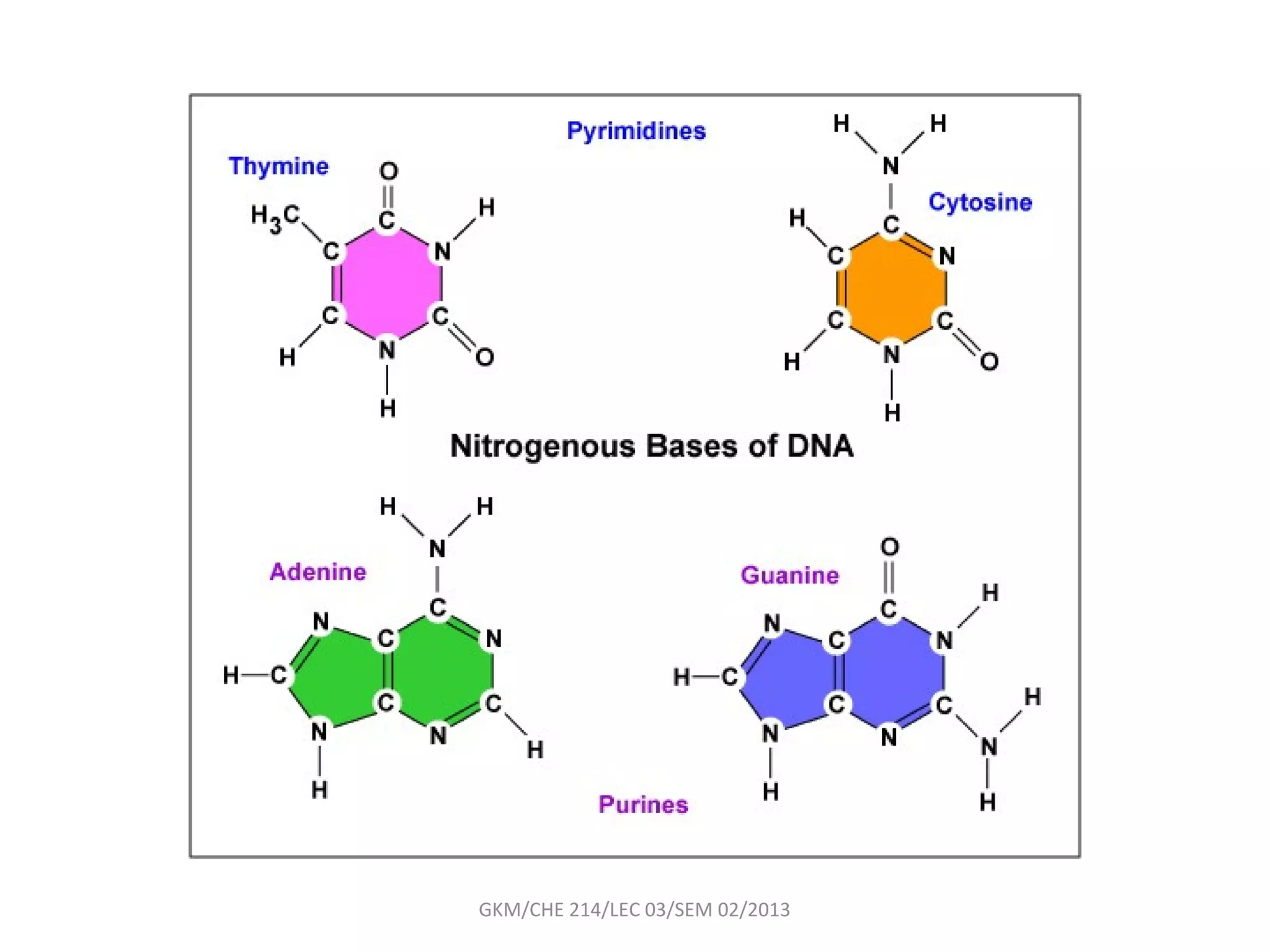
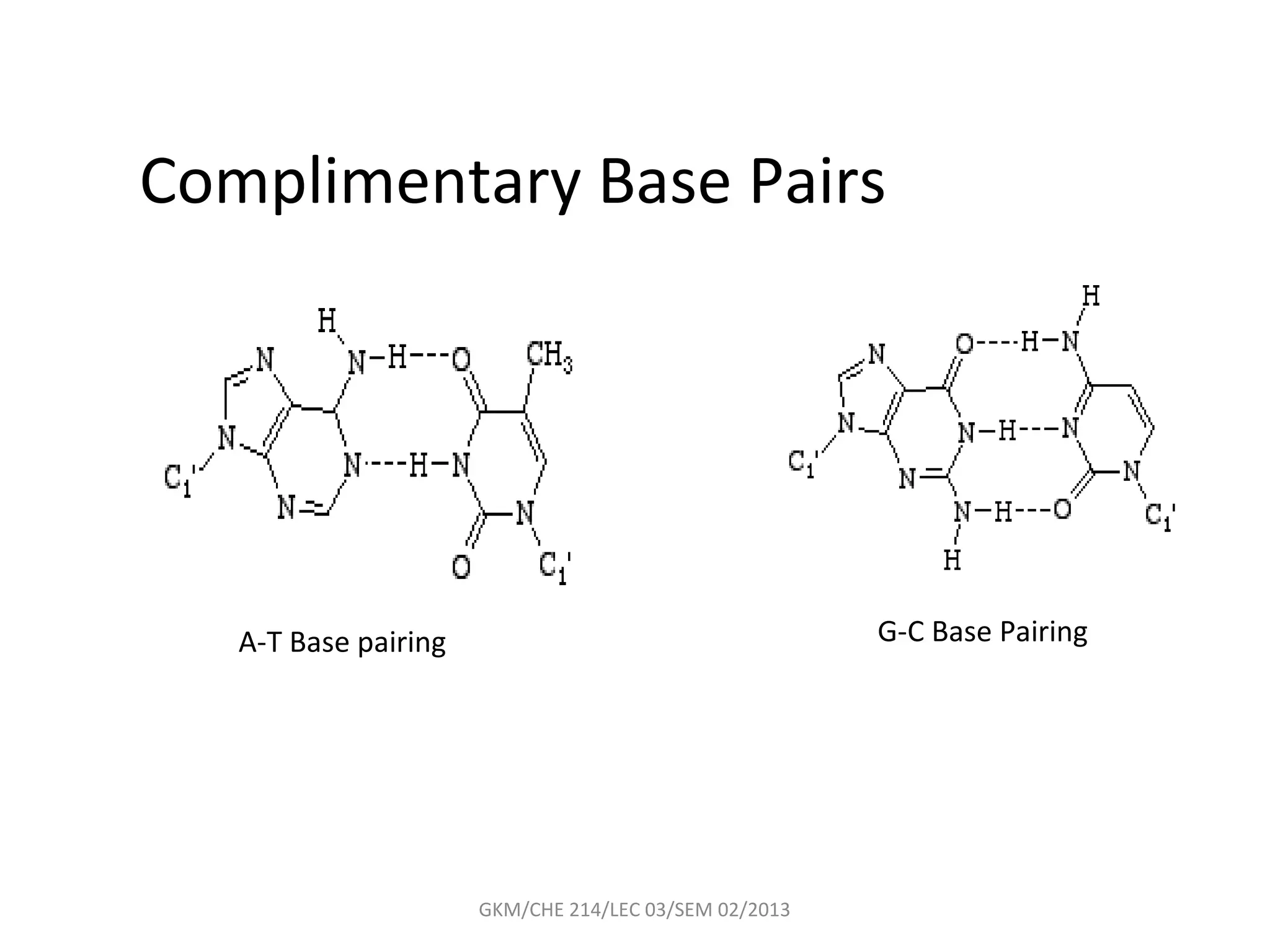
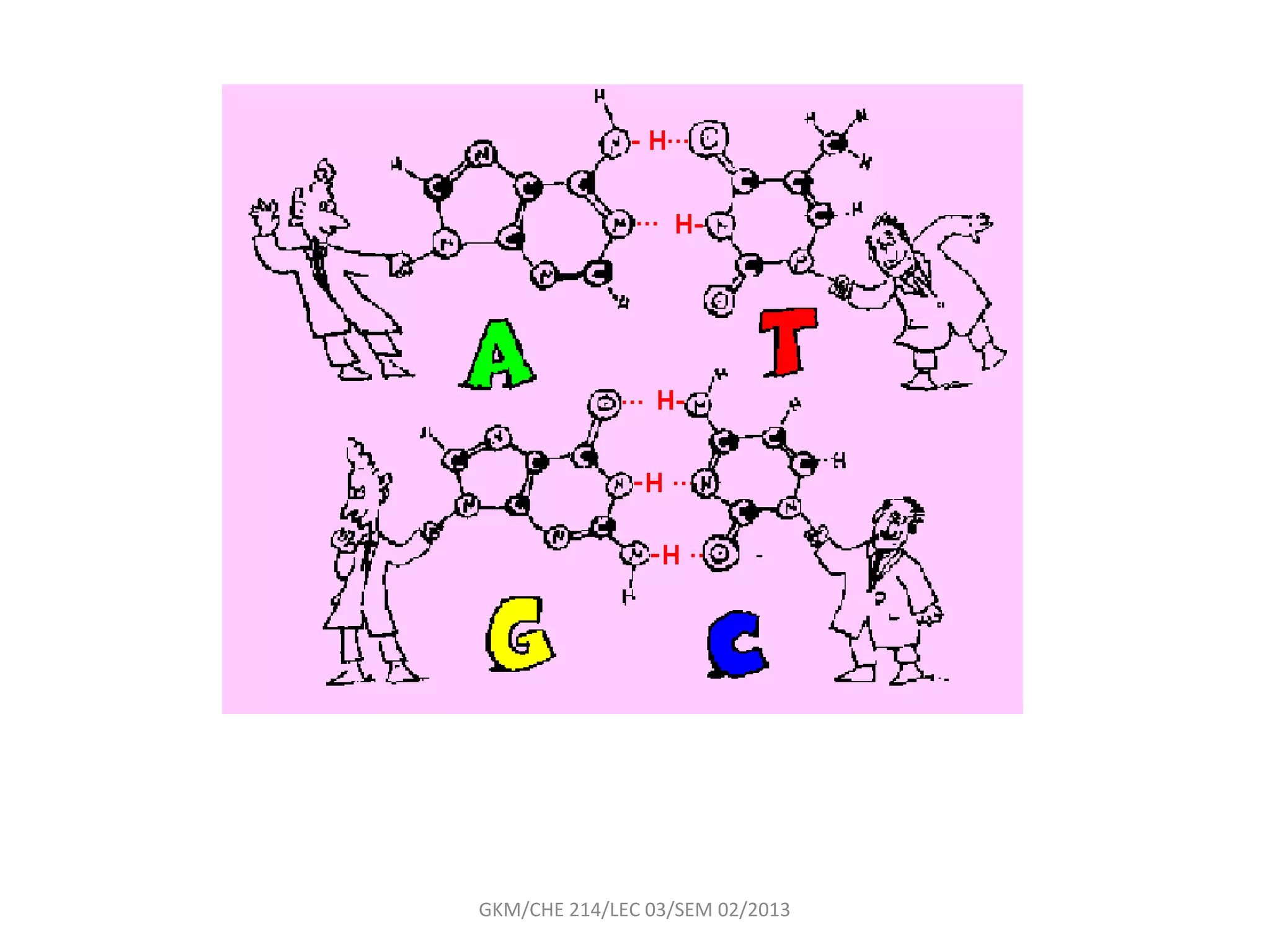
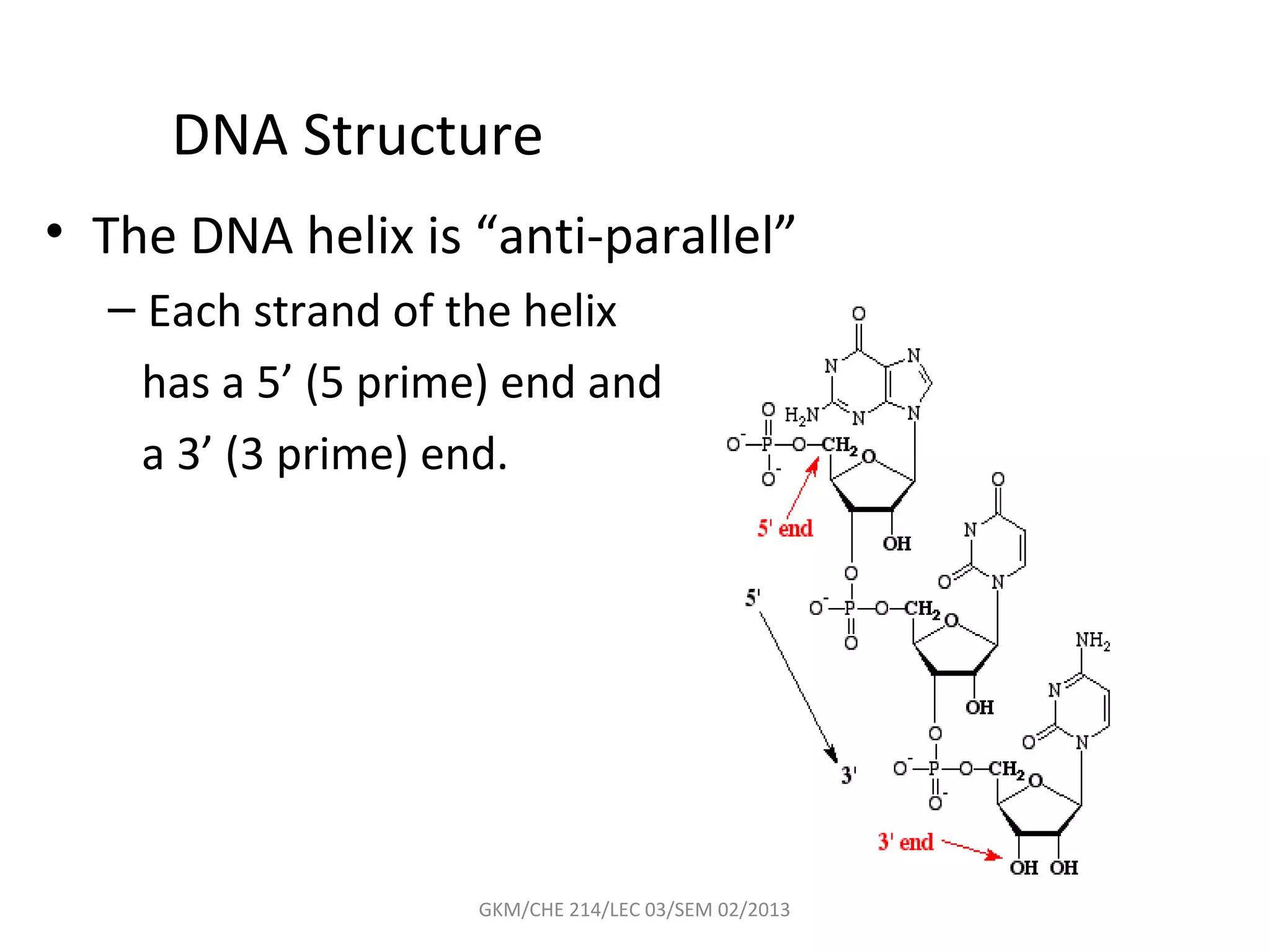
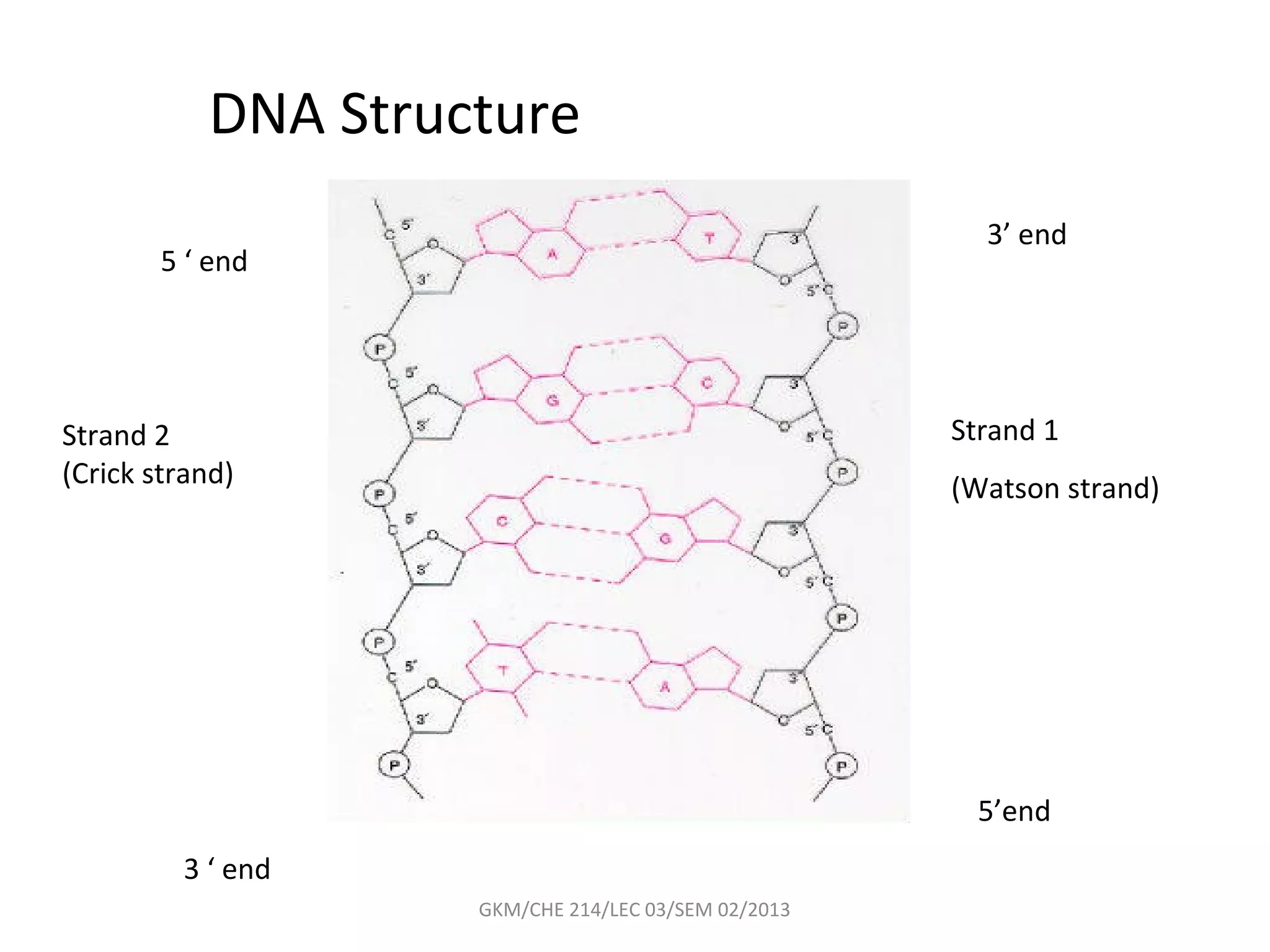
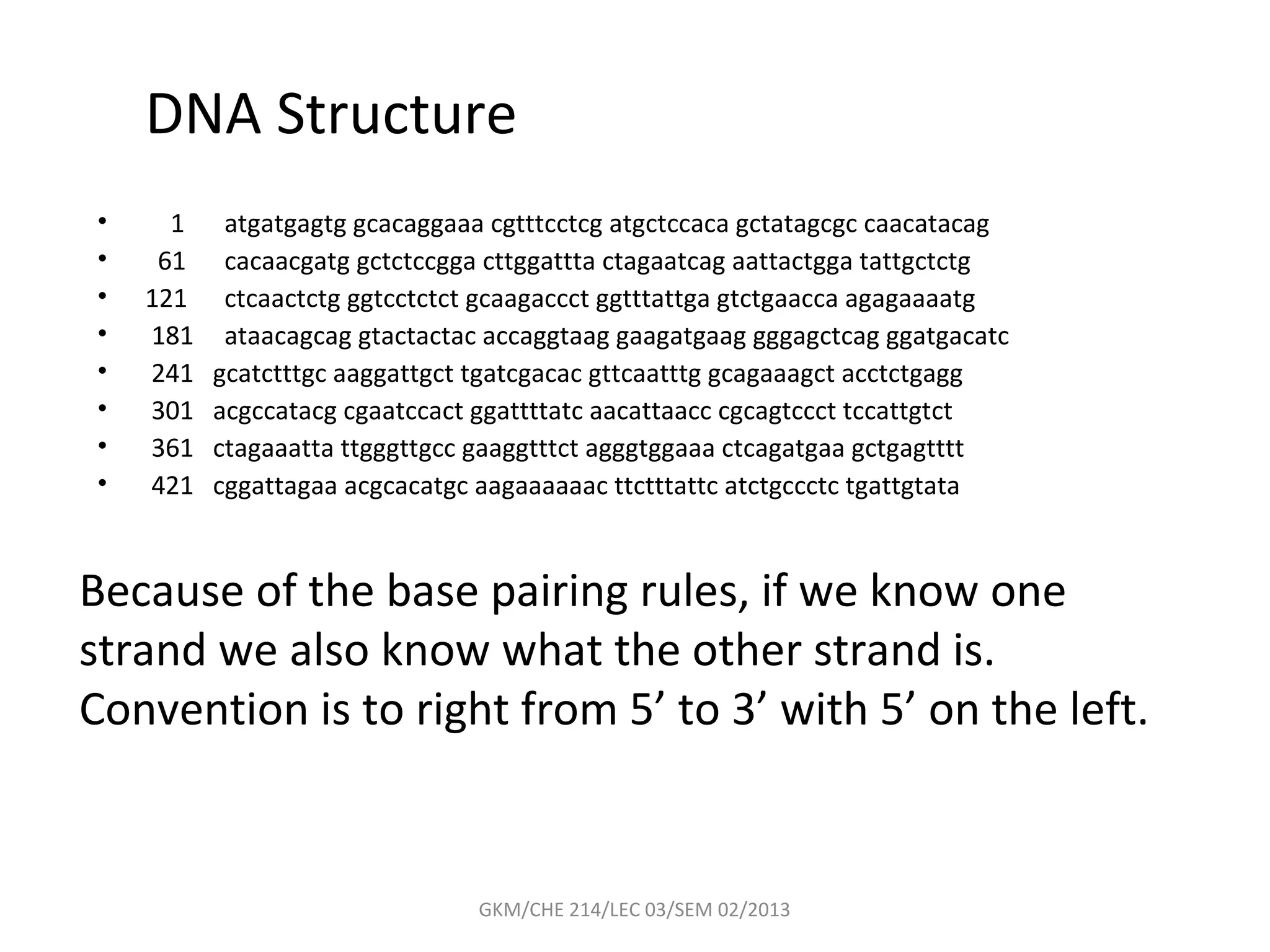
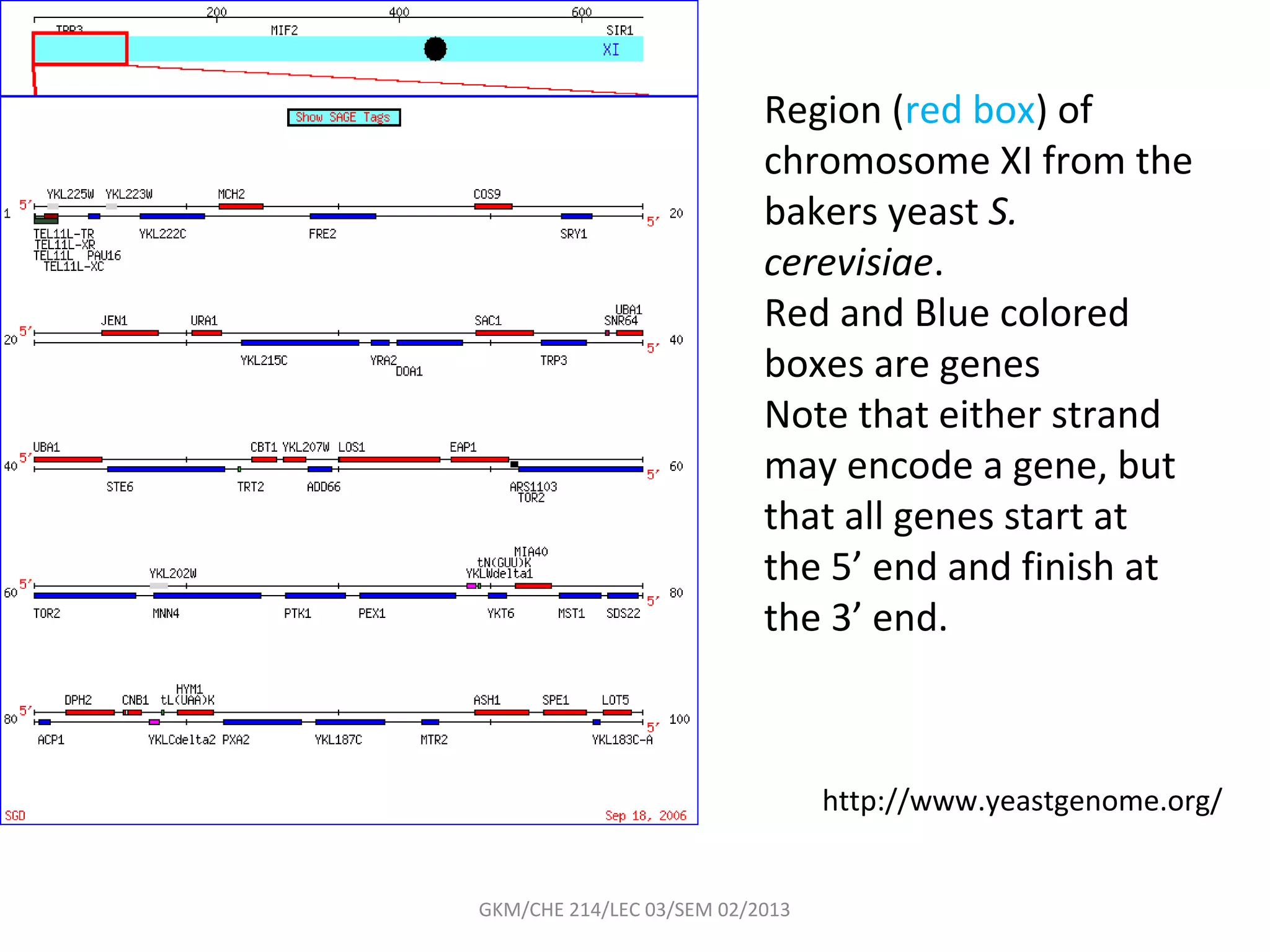
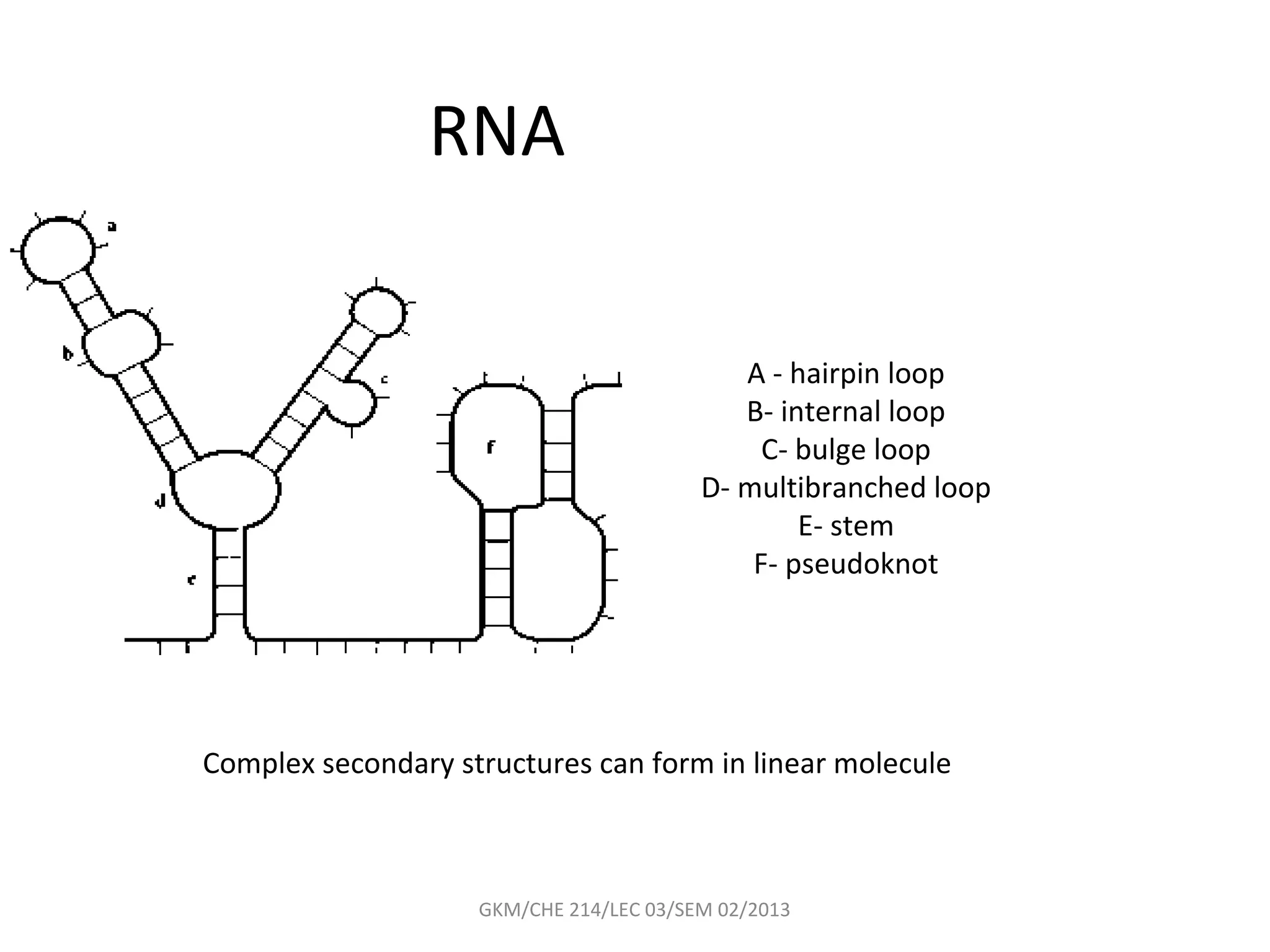
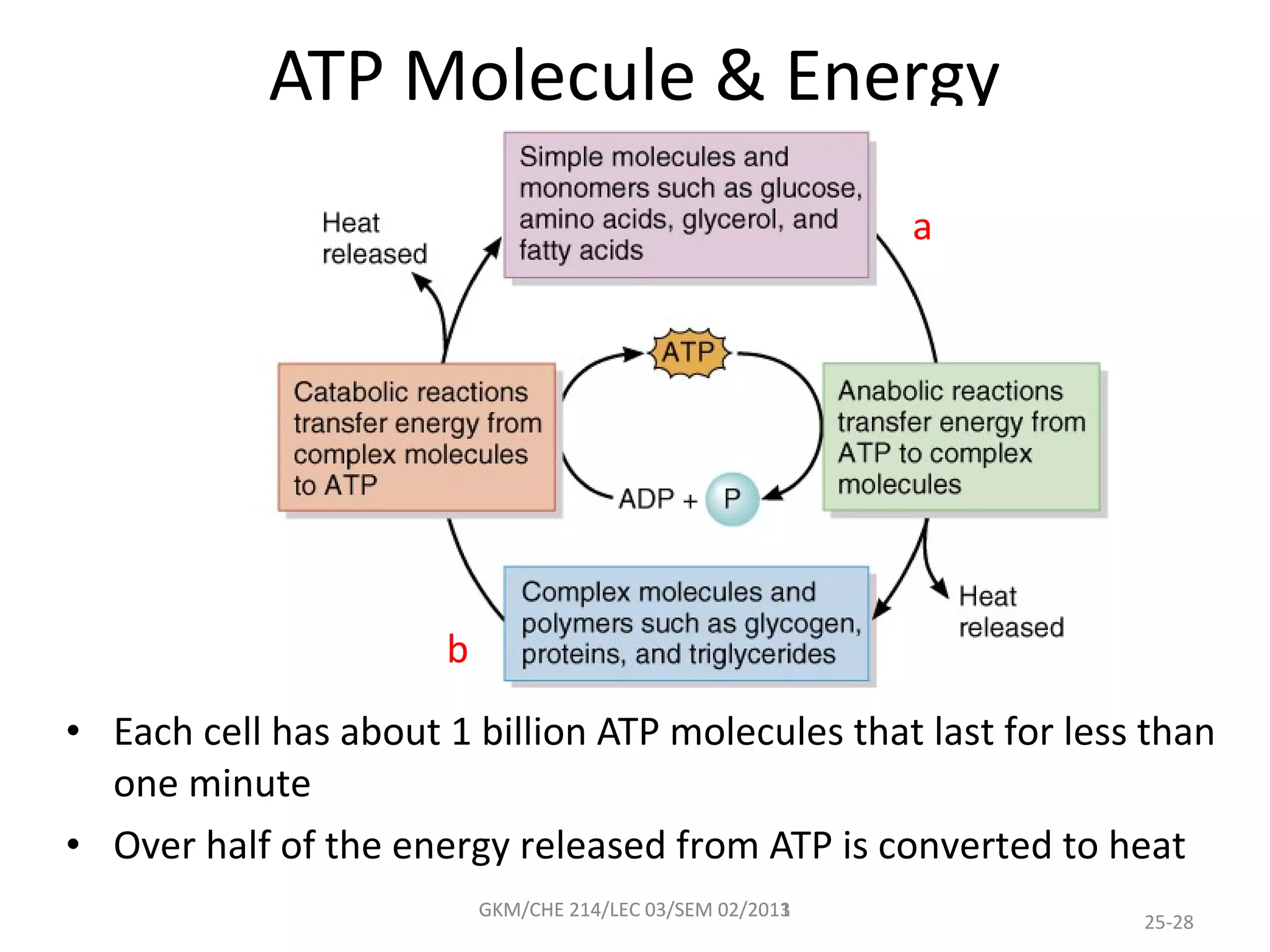
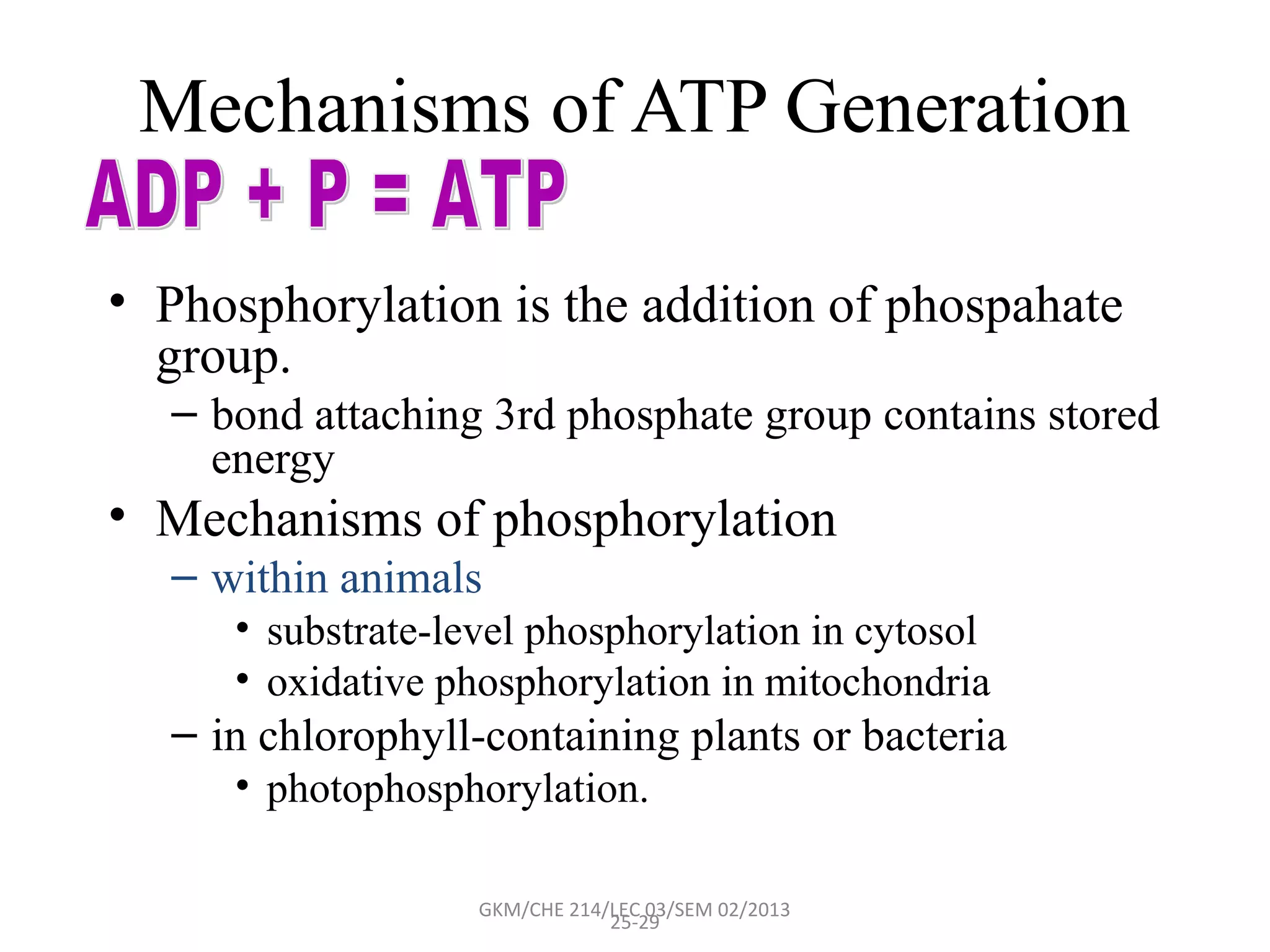
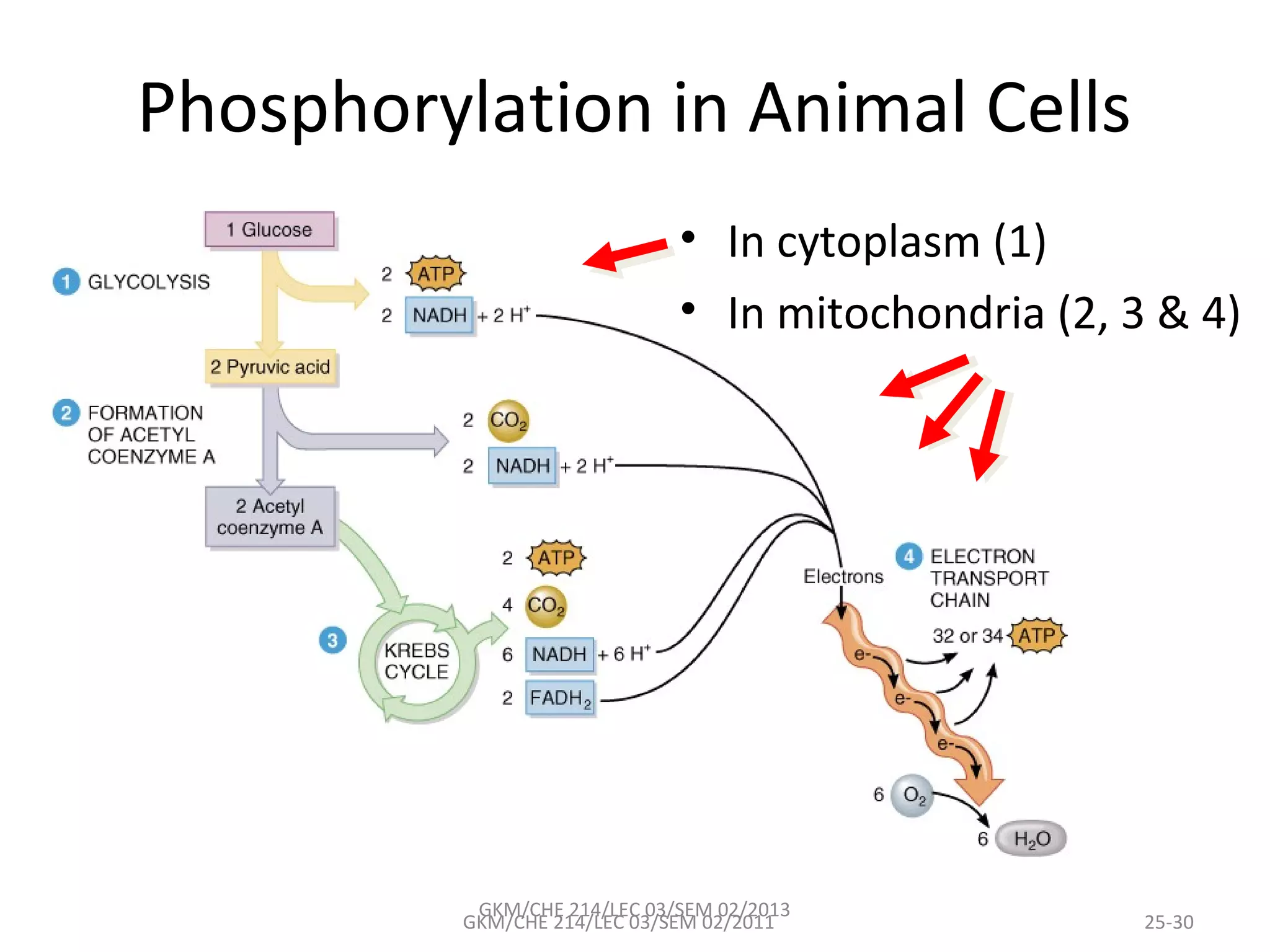
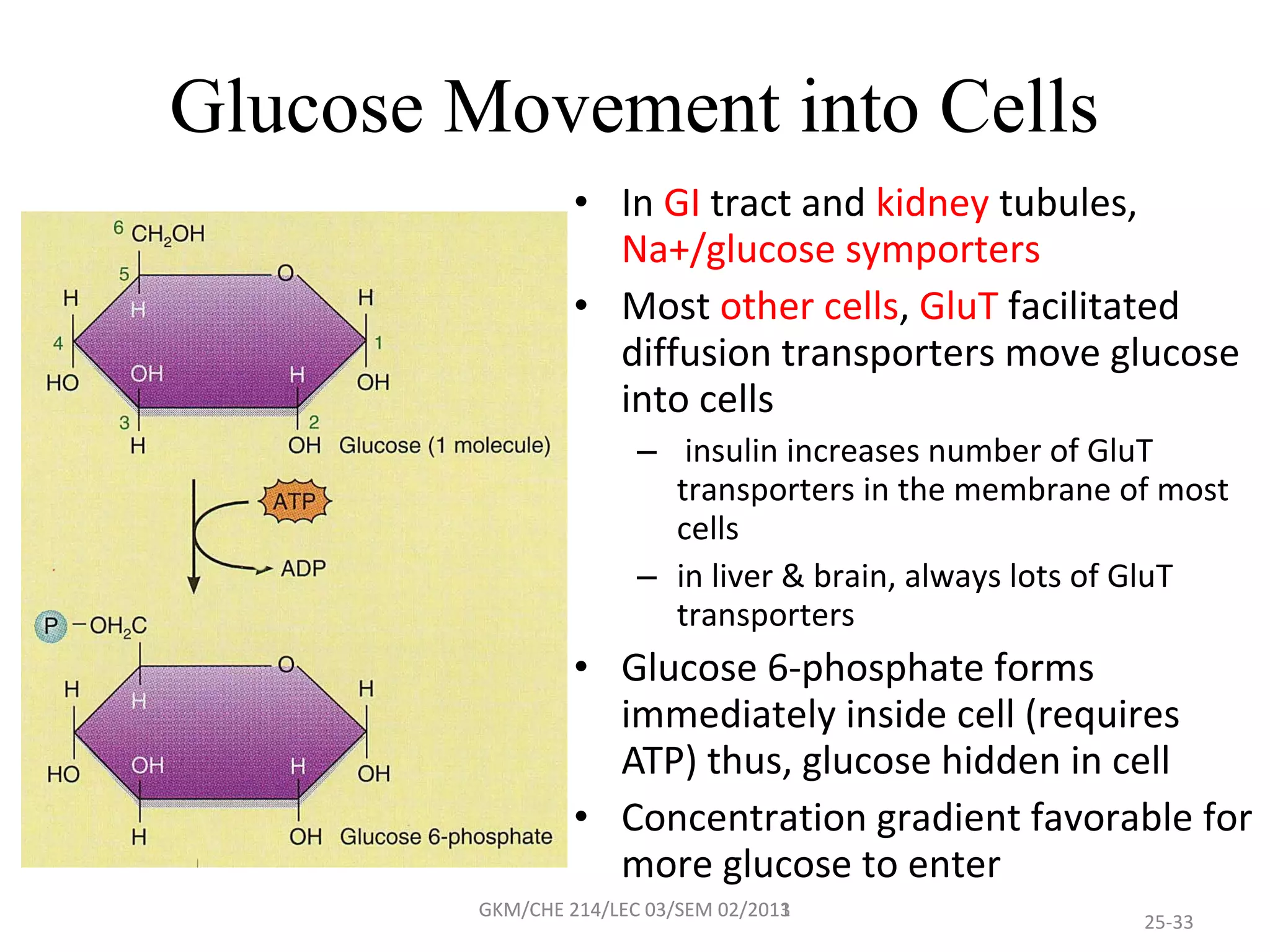
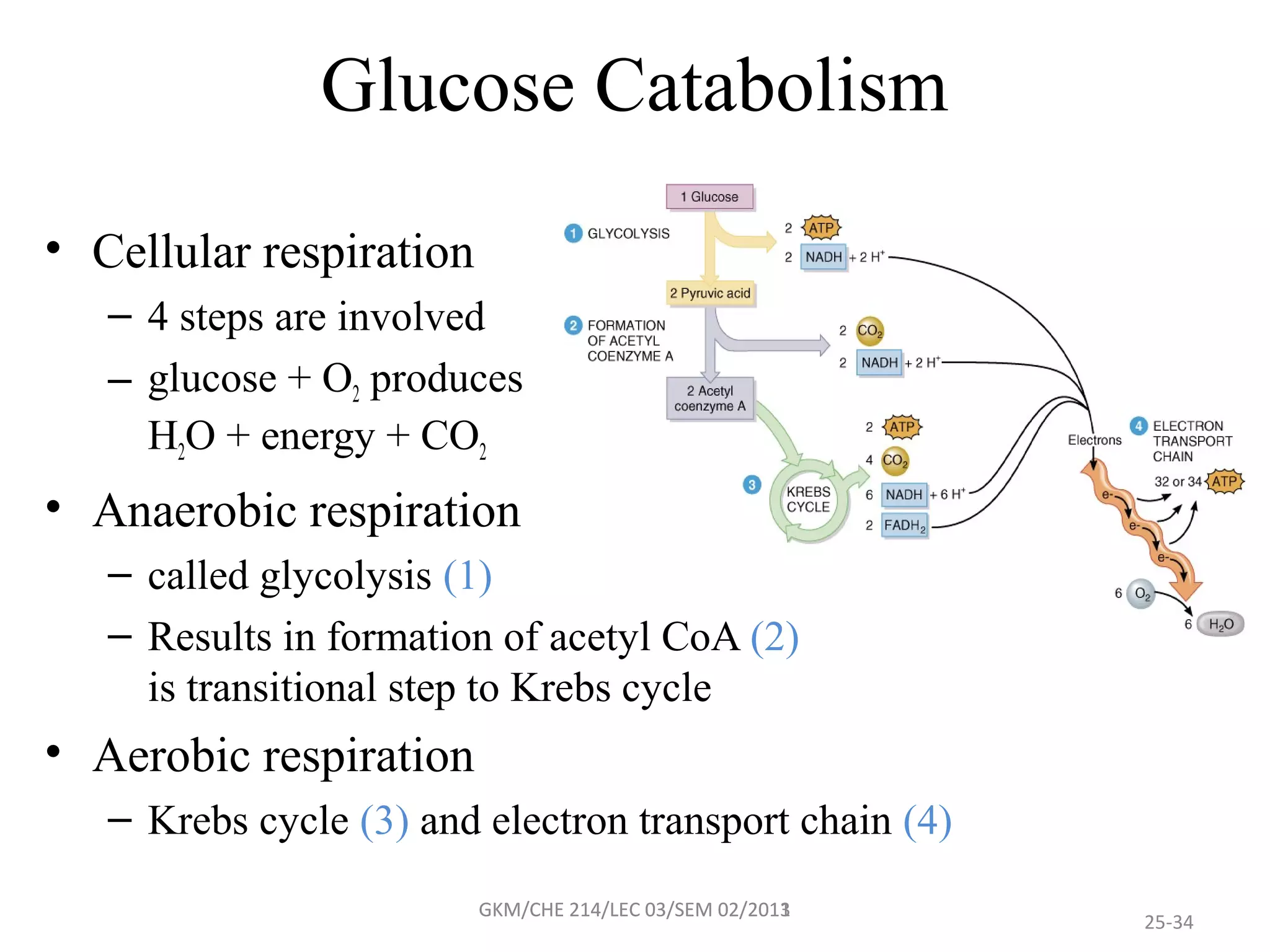
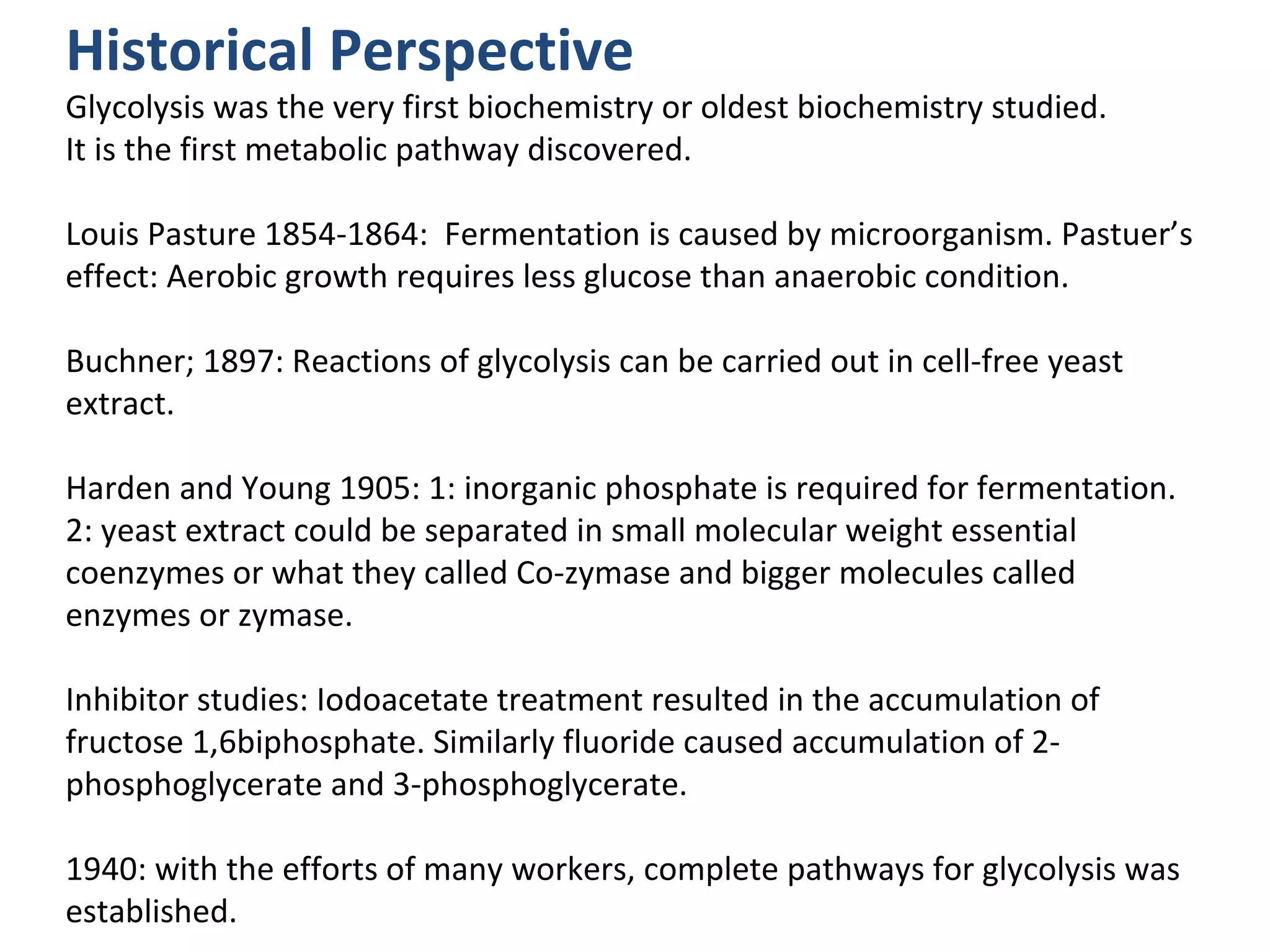
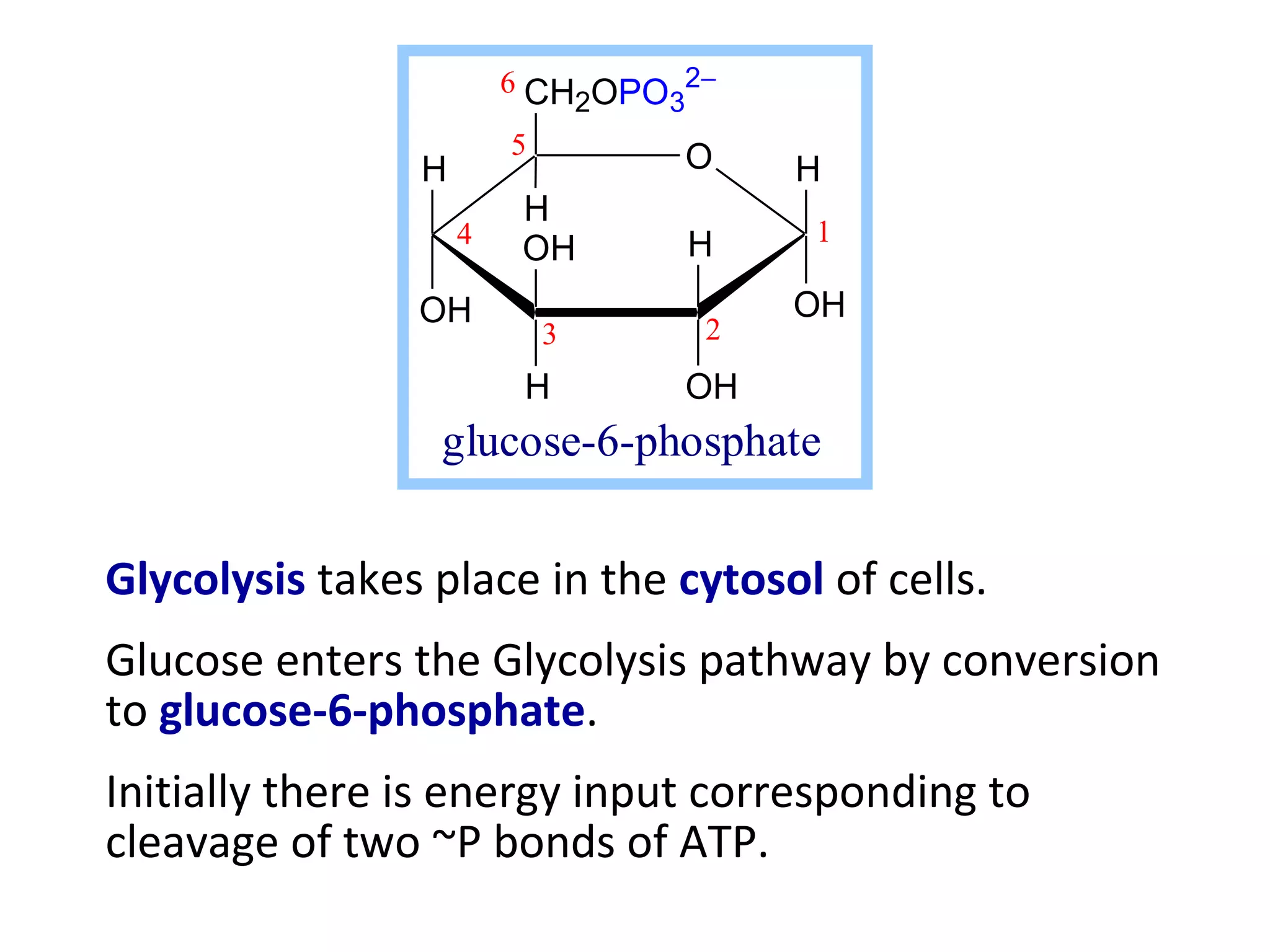
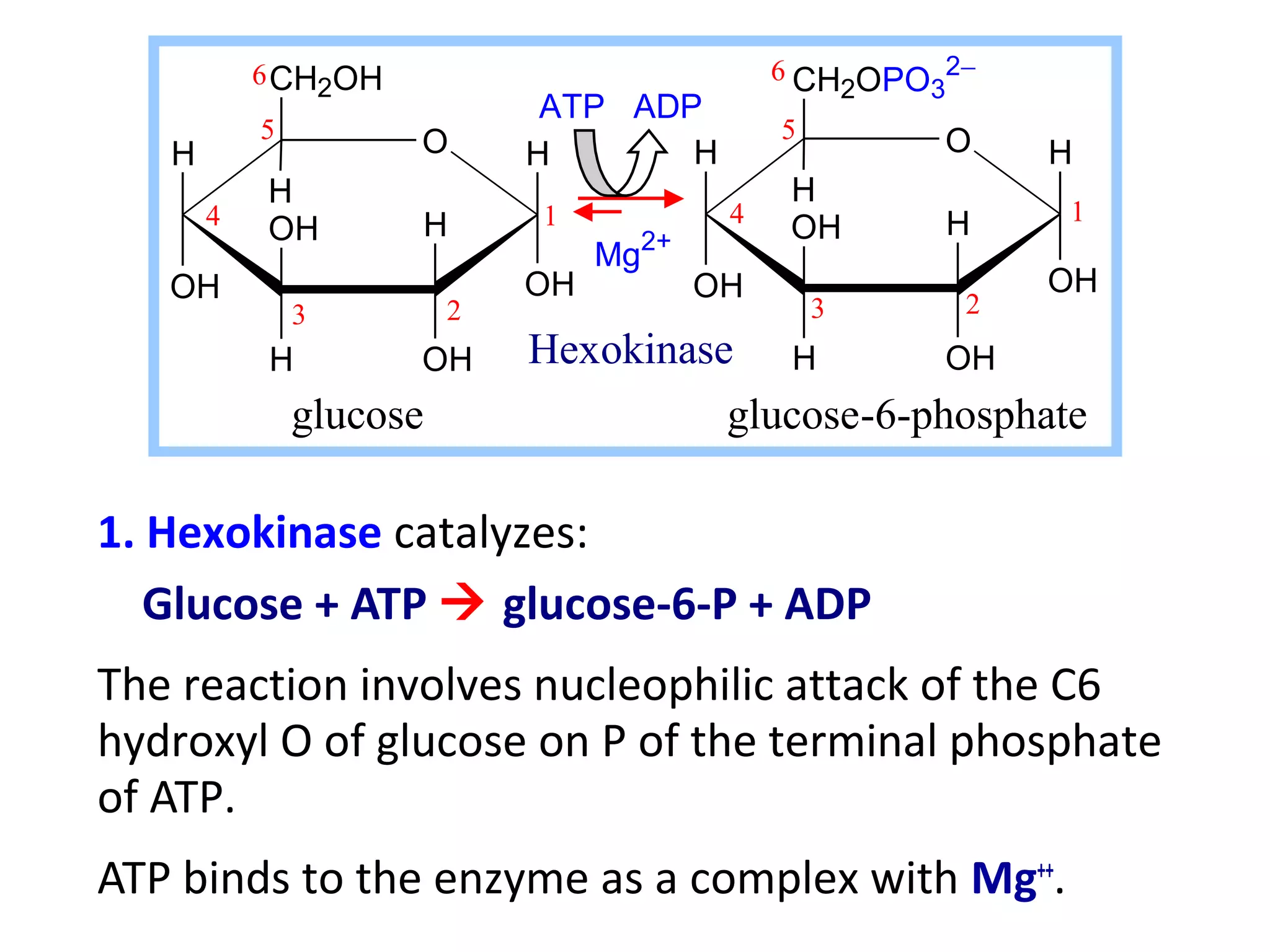
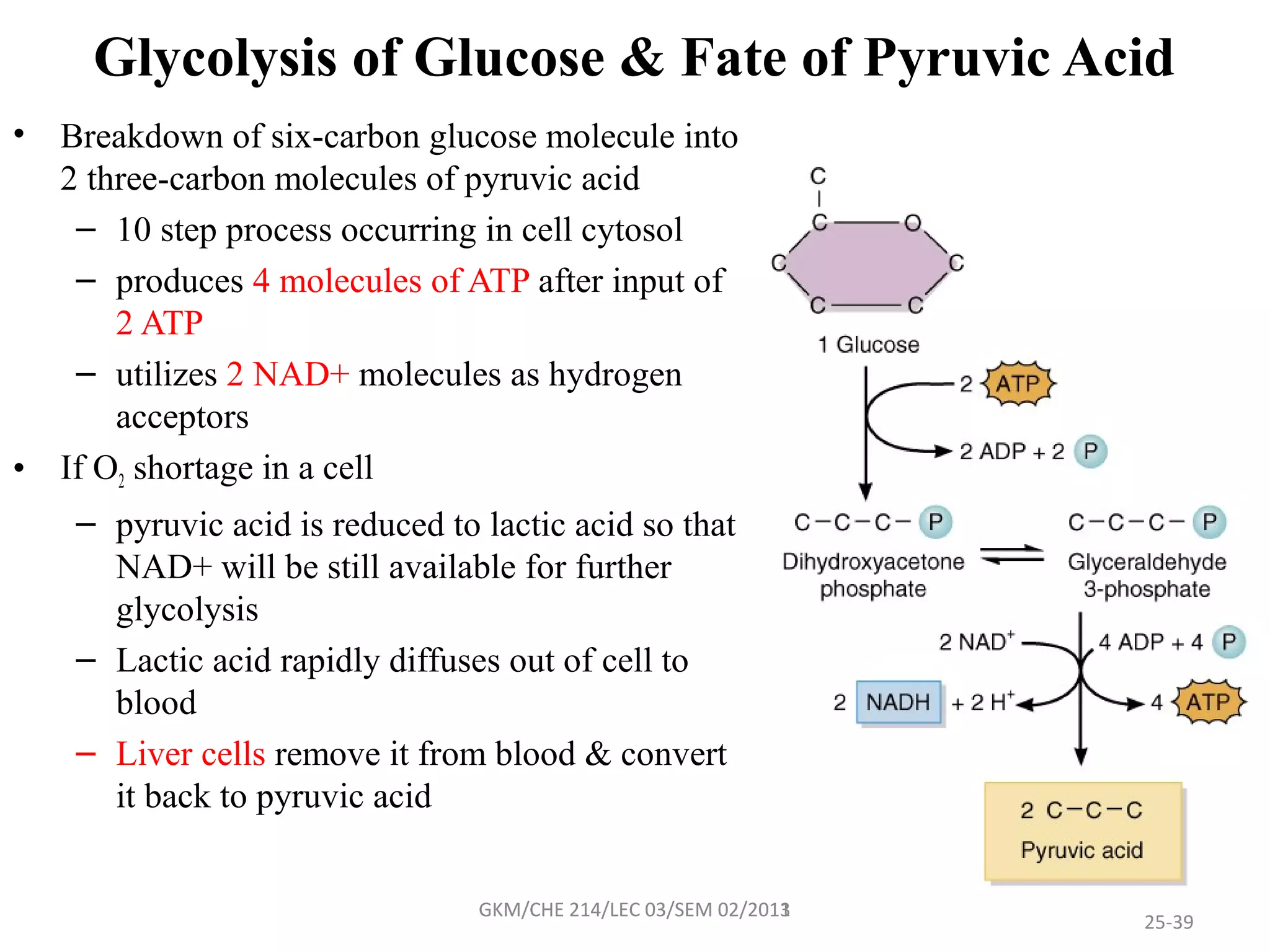
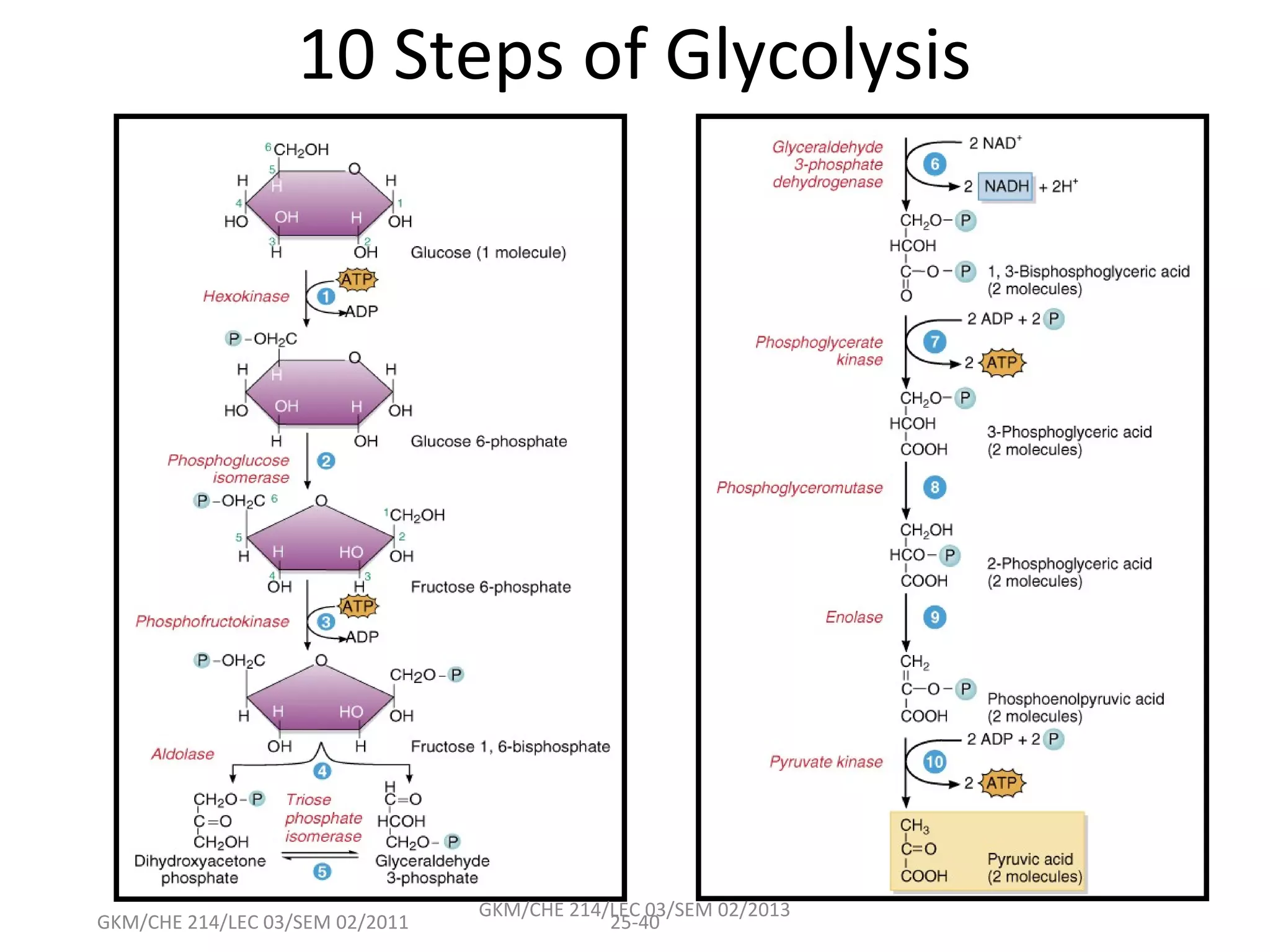
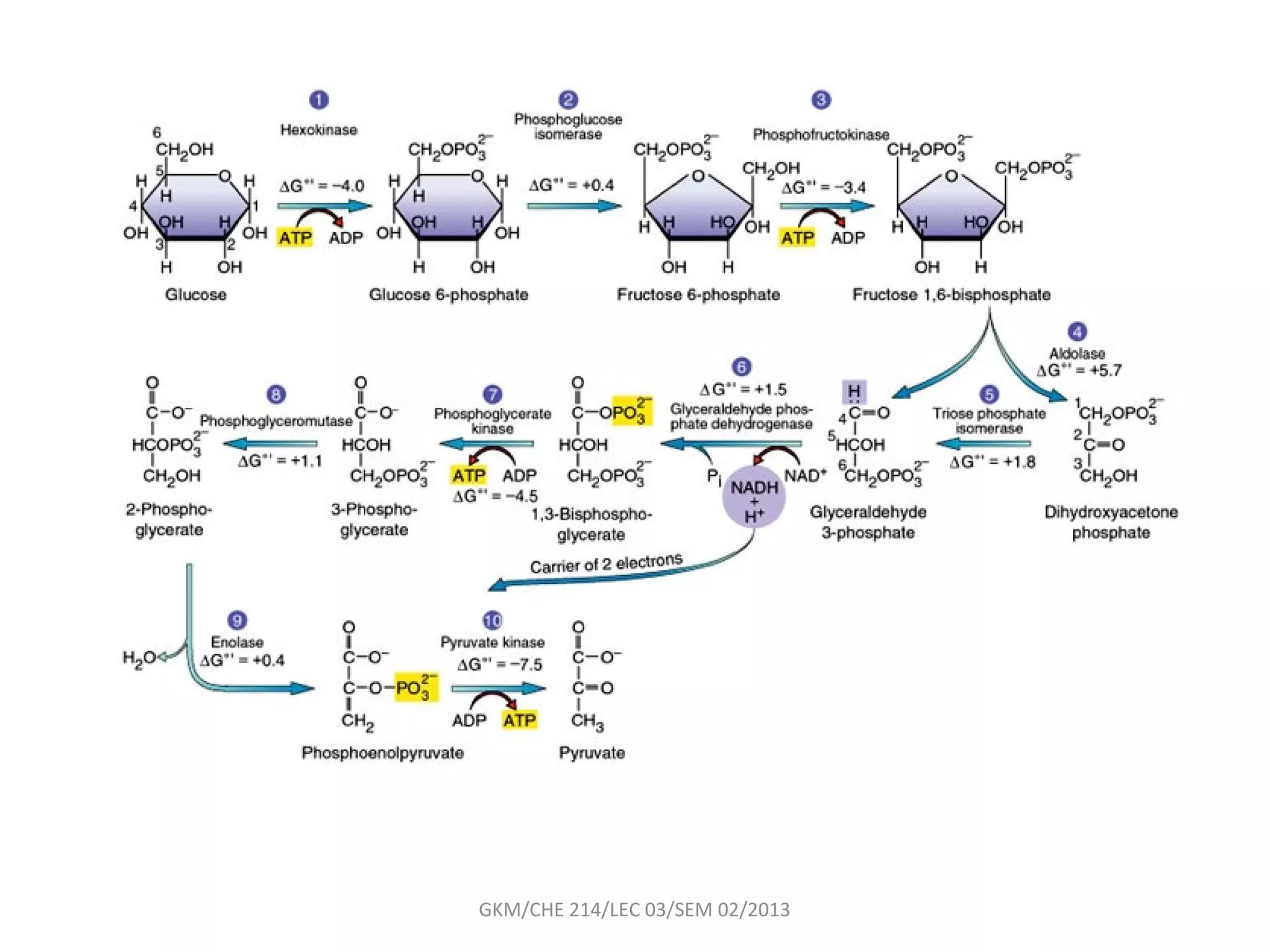
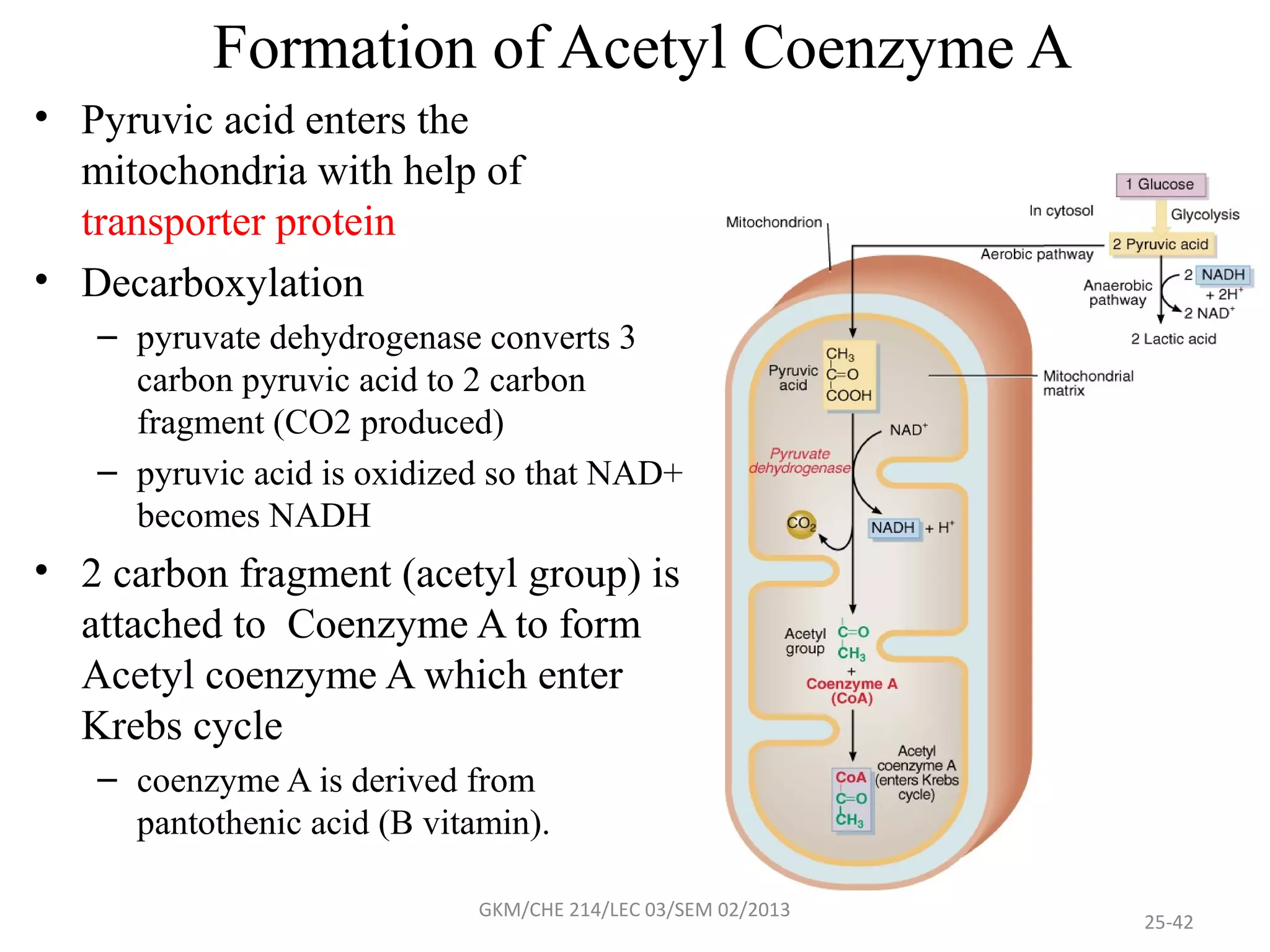
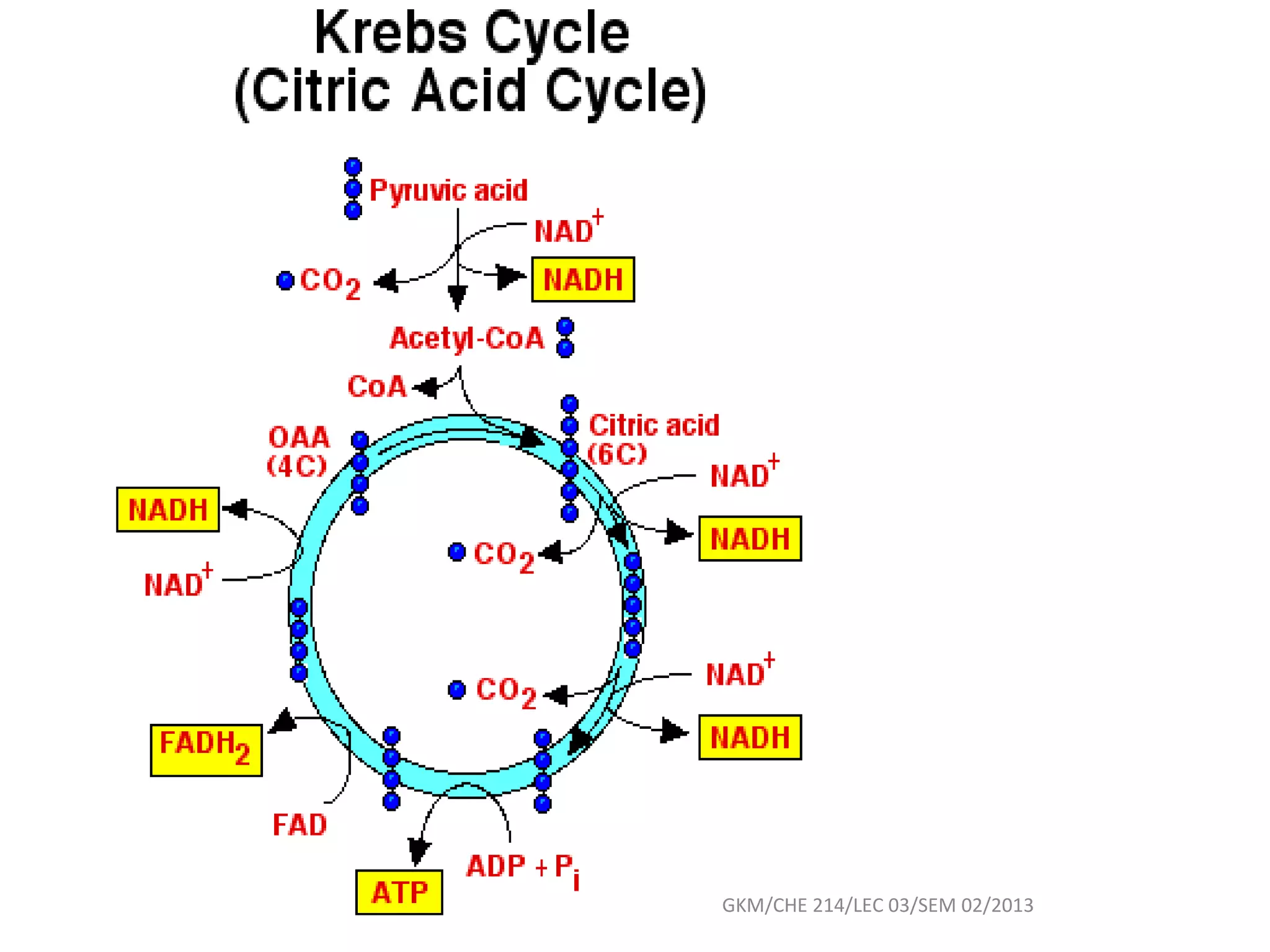
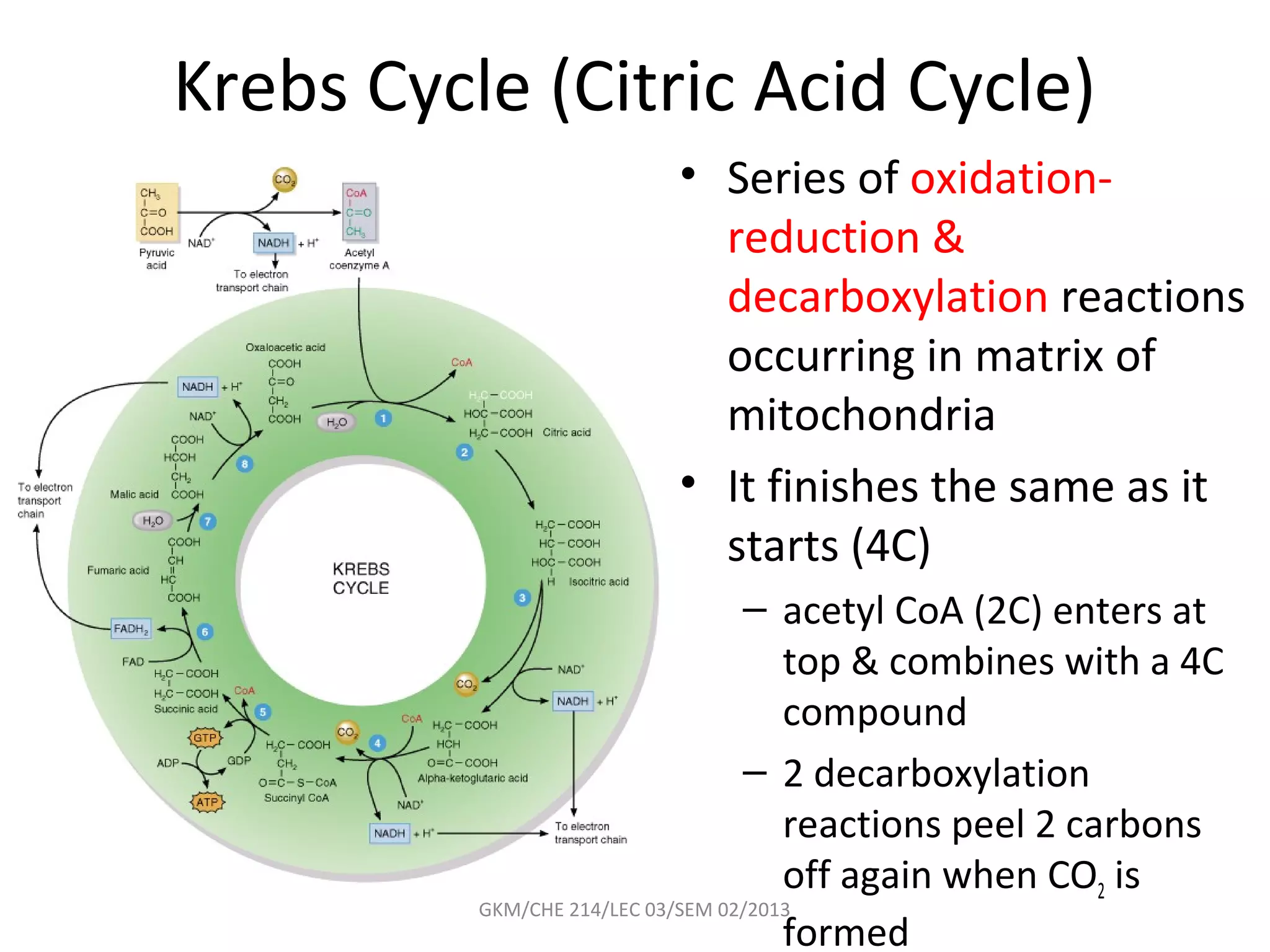
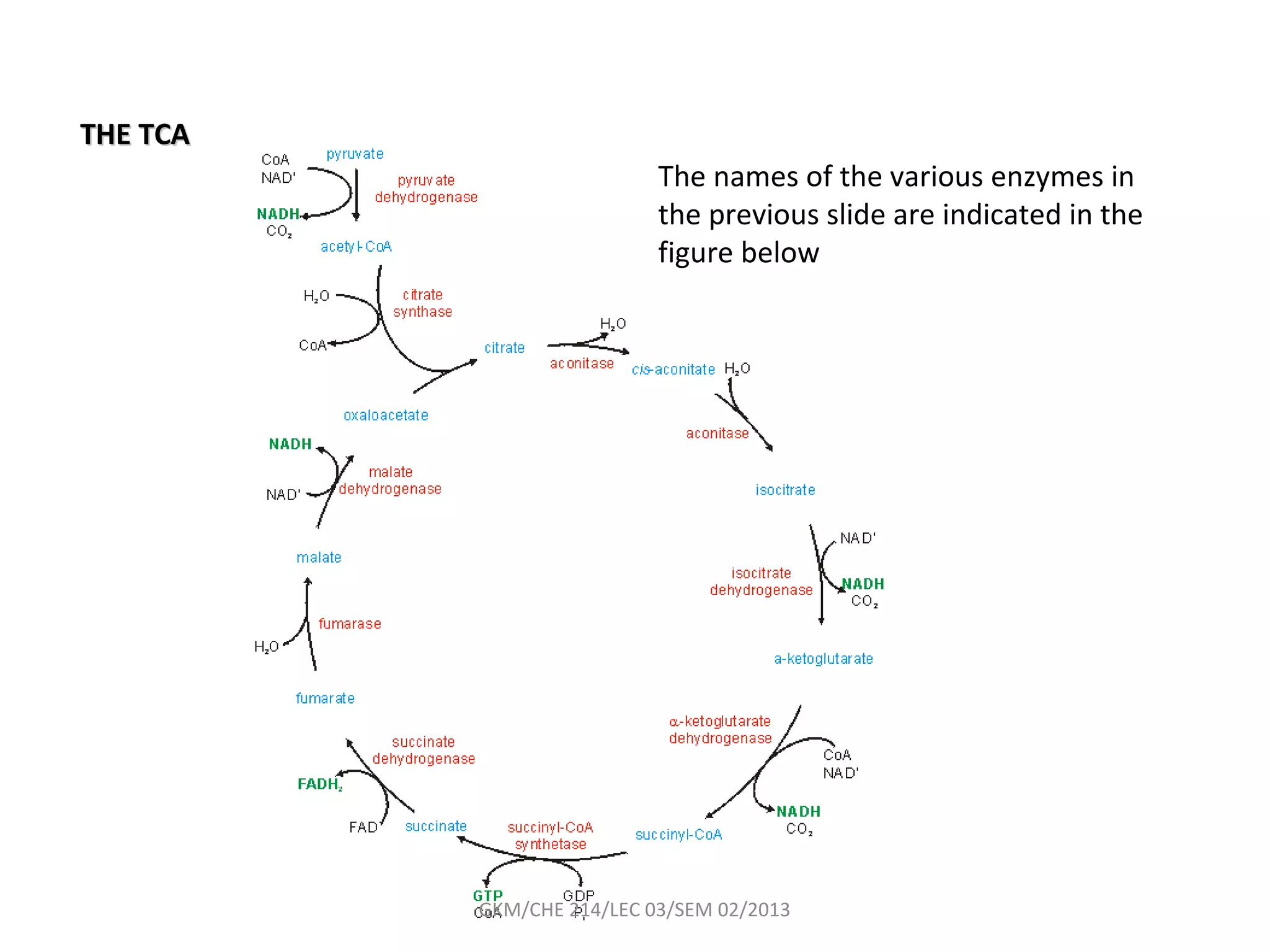
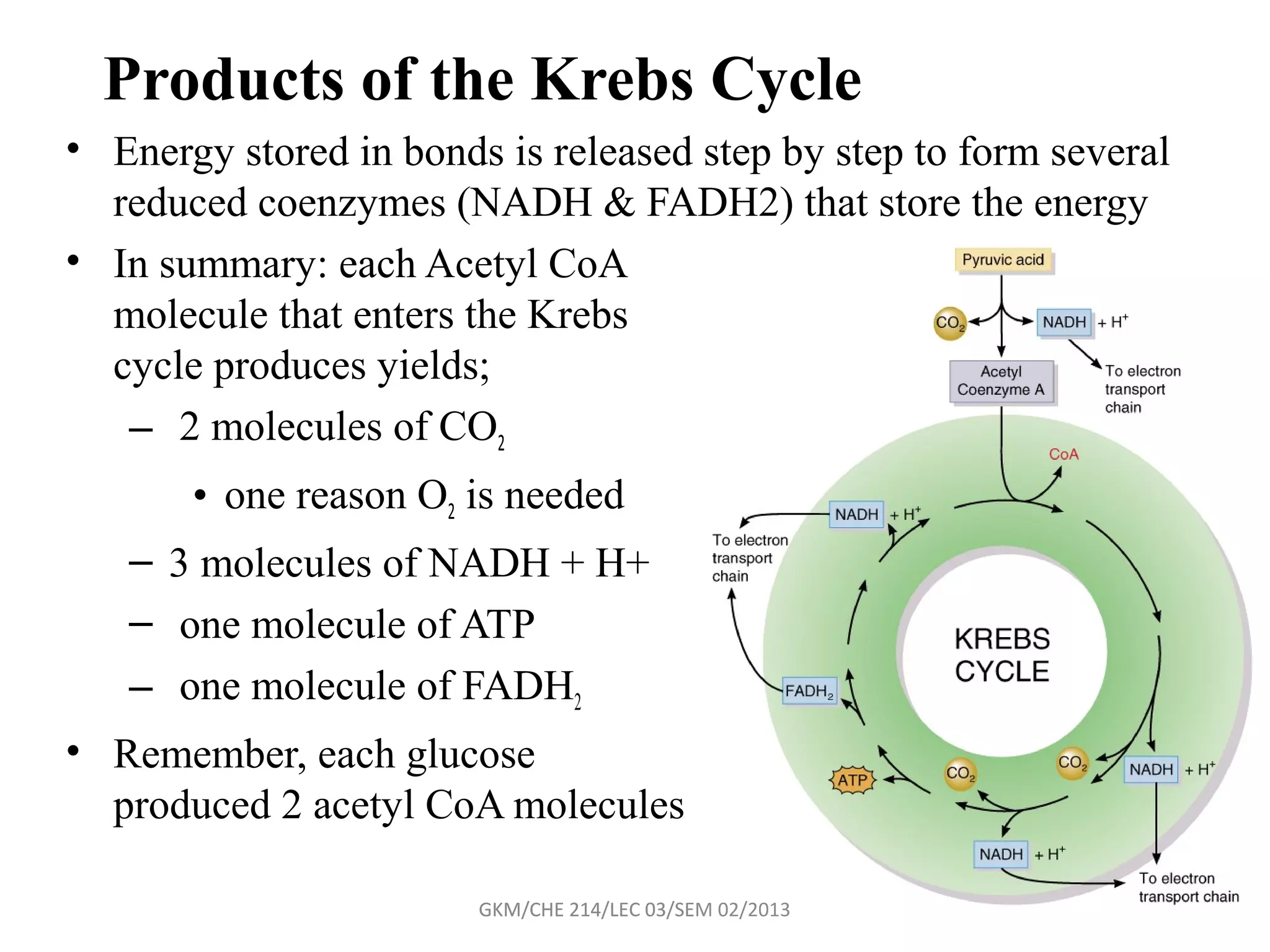
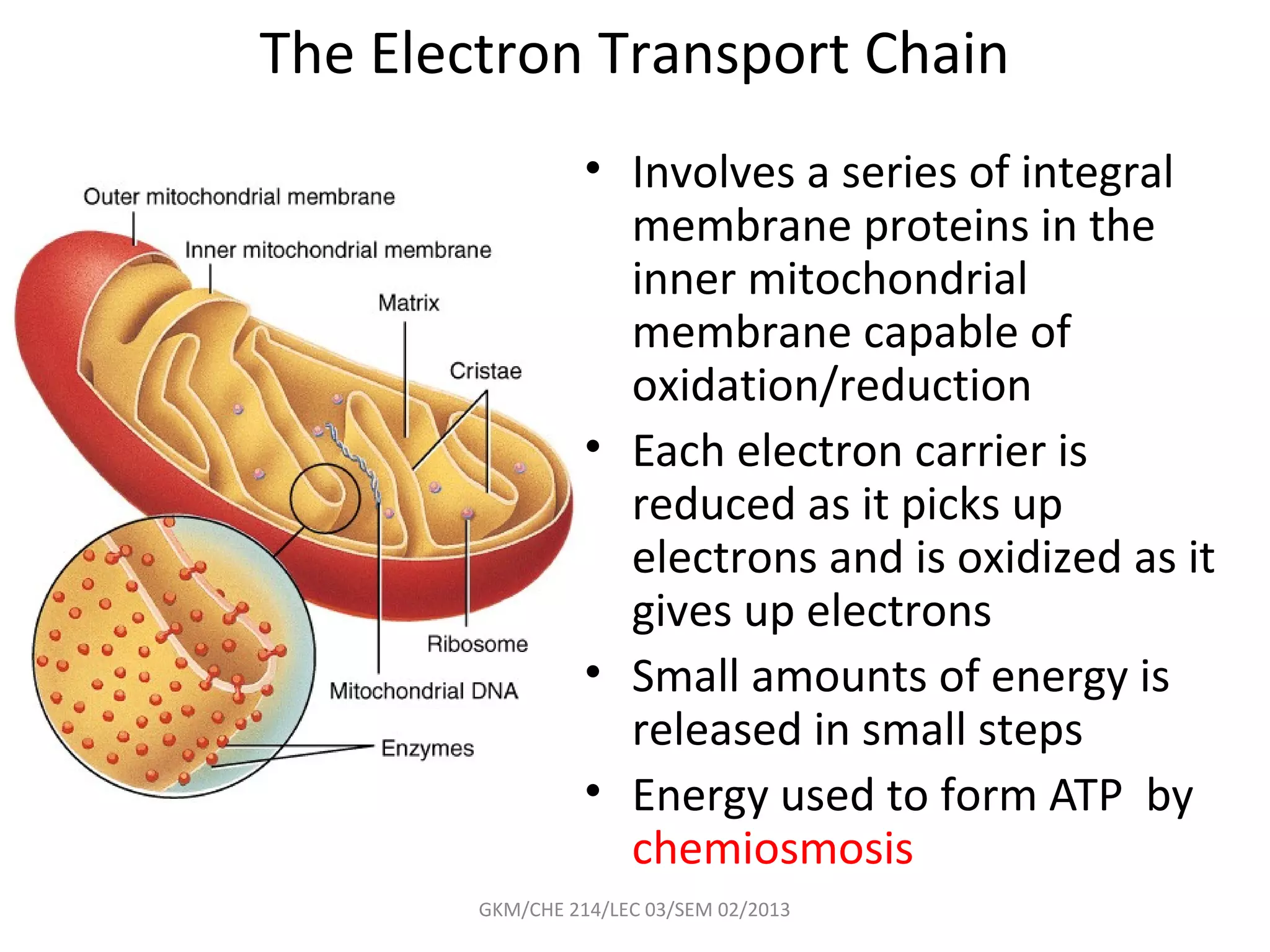
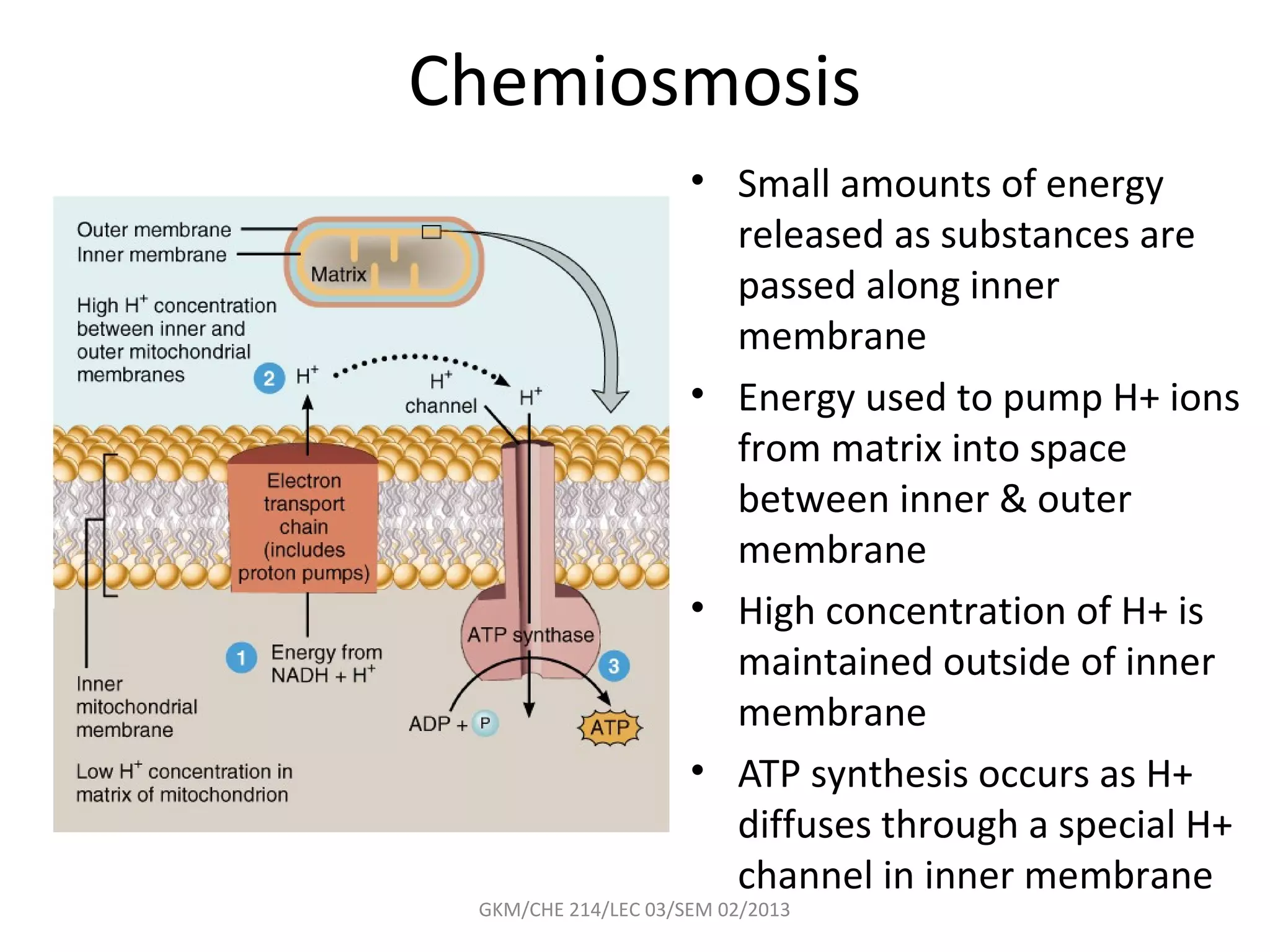
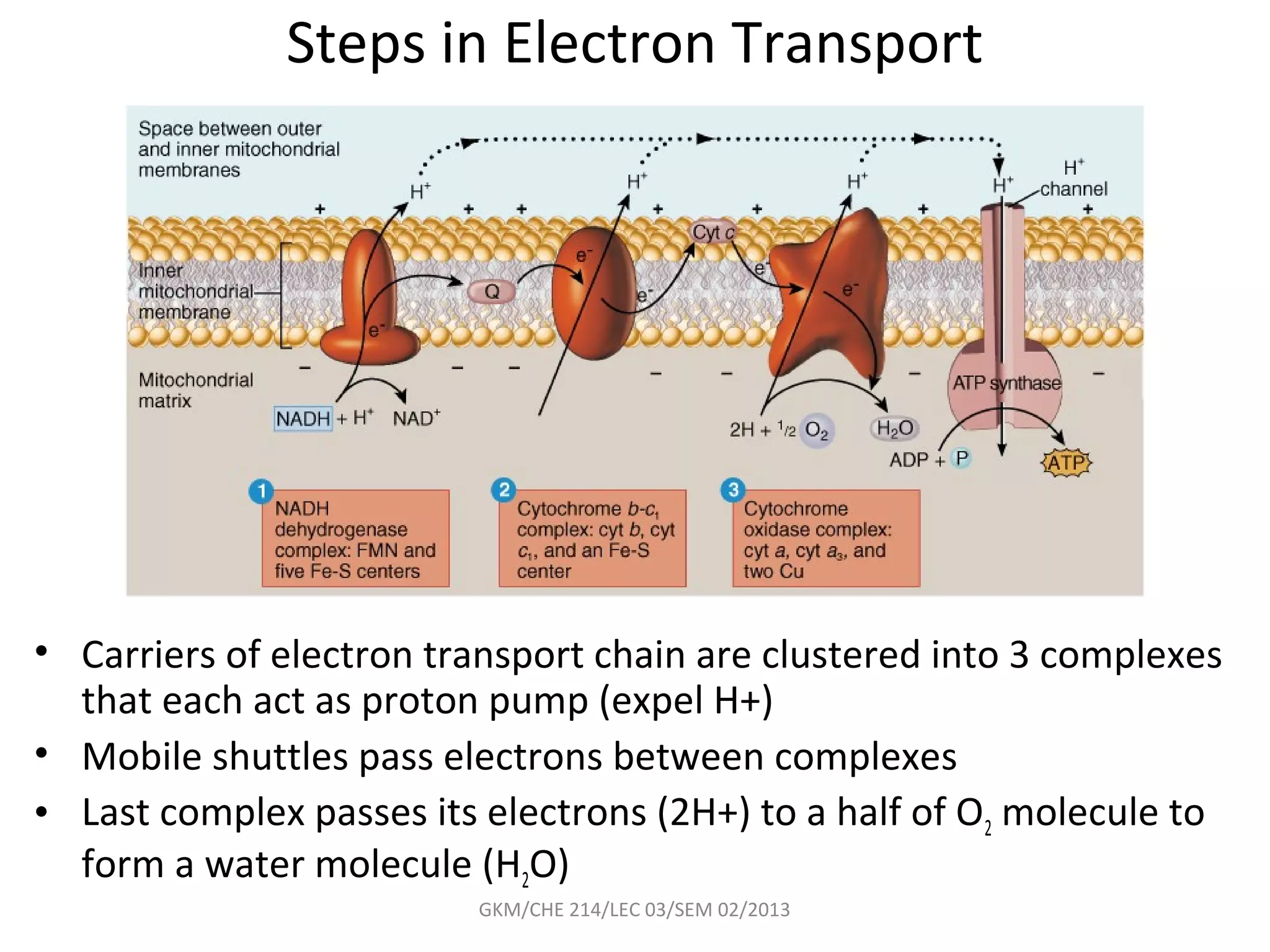
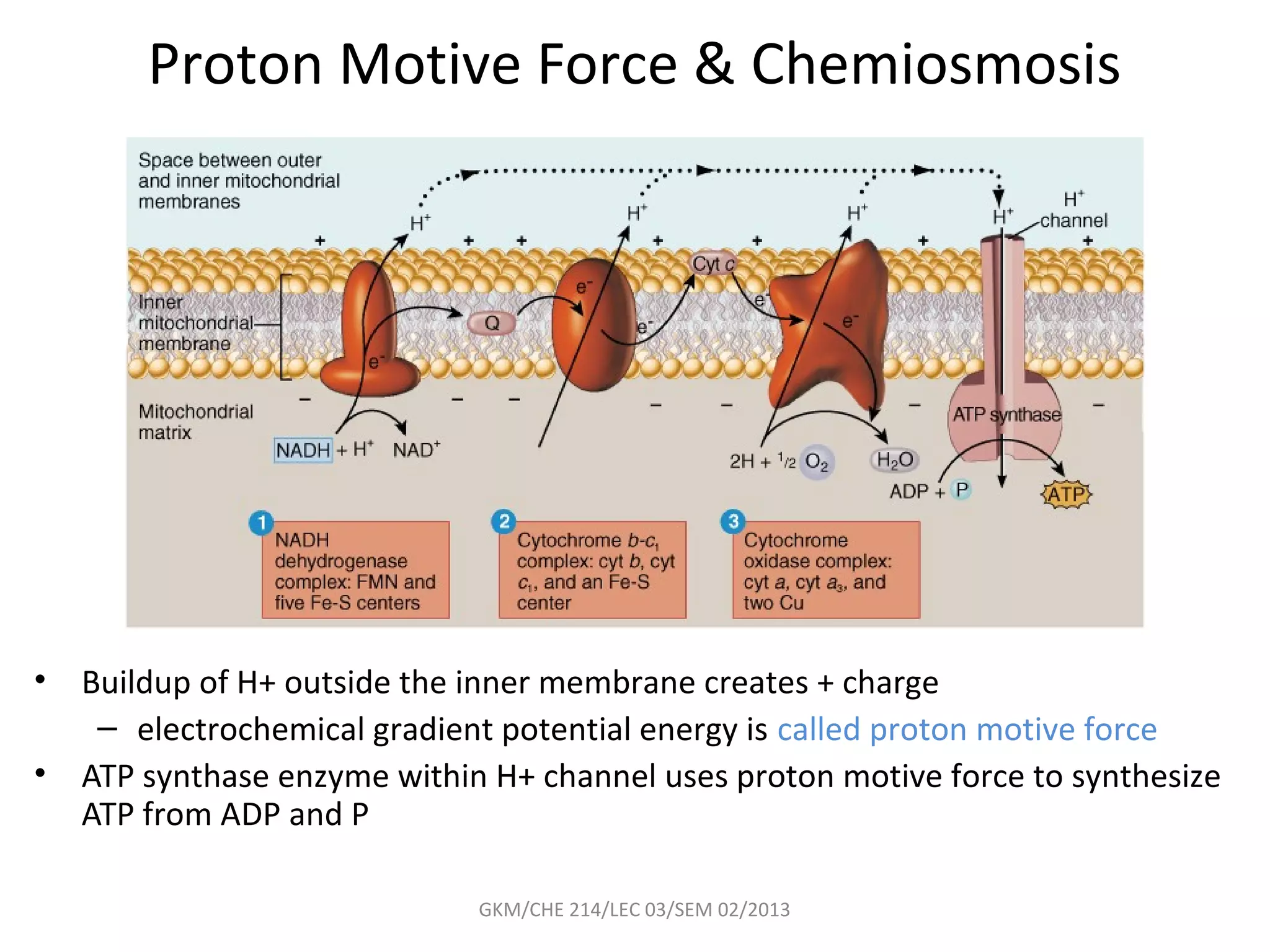
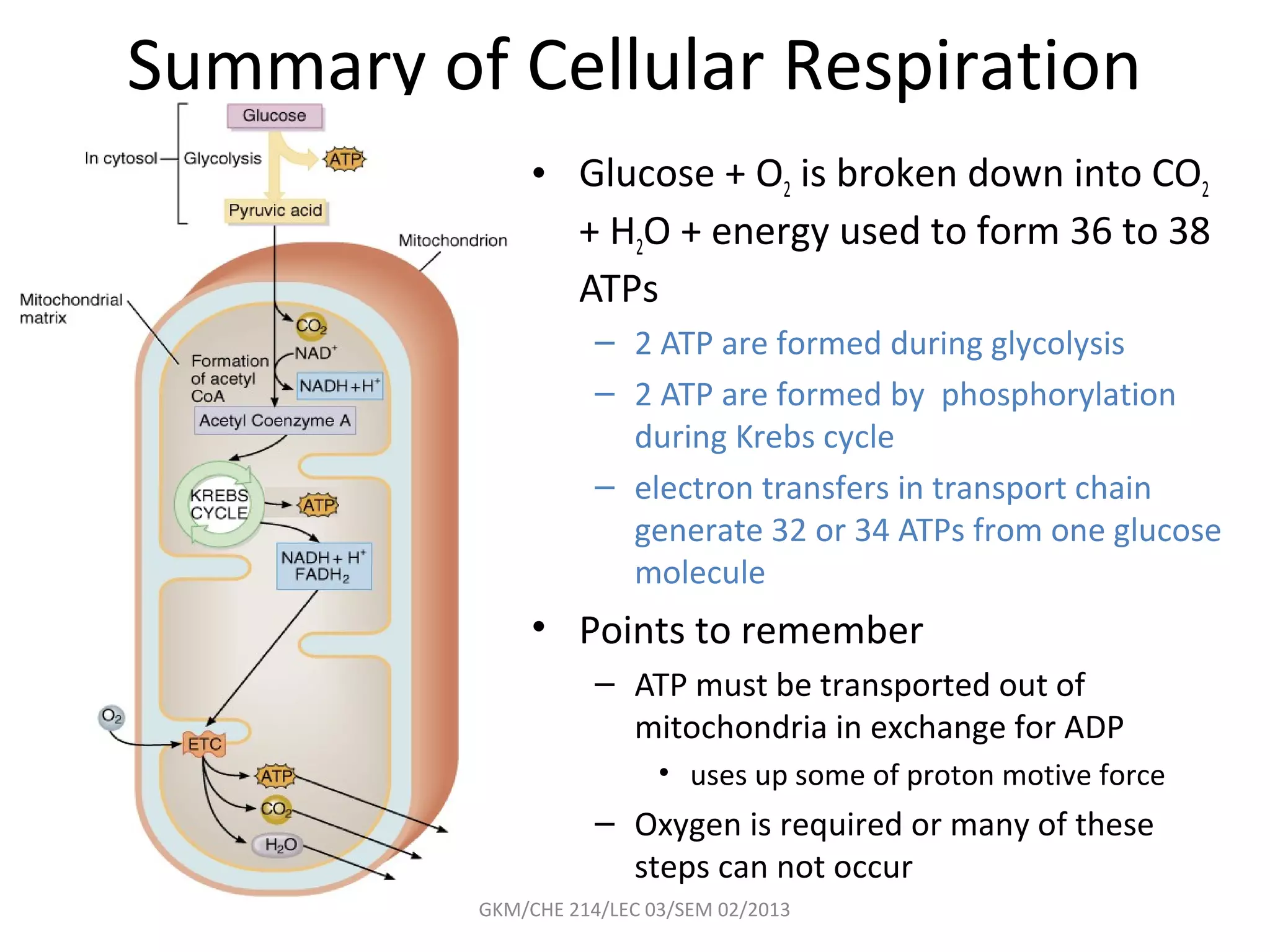
The document summarizes a lecture on nucleic acids and bioenergetics. It discusses the basics of DNA and RNA, including their components, structures, and functions. It covers DNA structure including the double helix formation and base pairing. It also discusses RNA types and functions. The second part of the lecture covers bioenergetics, including ATP generation through phosphorylation, carbohydrate metabolism like glycolysis, and historical discoveries around glycolysis.