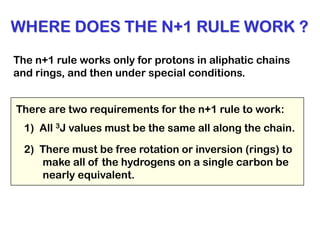
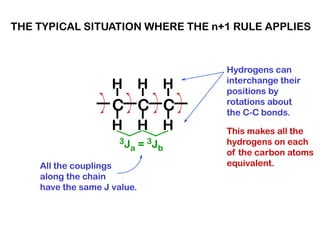
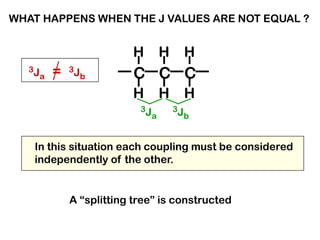
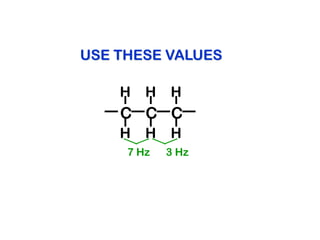
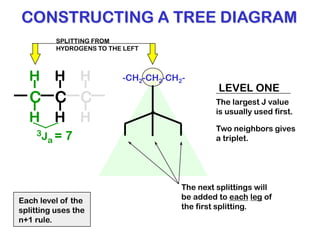
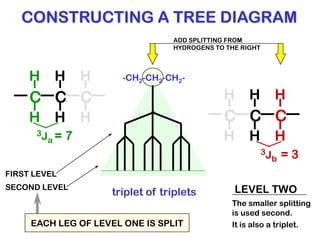
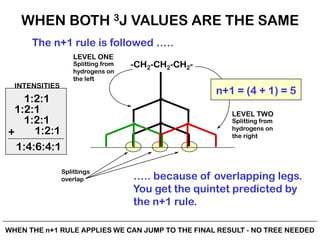
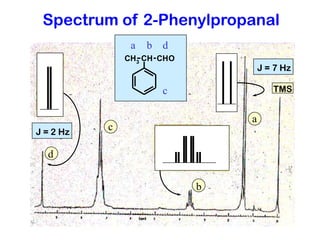
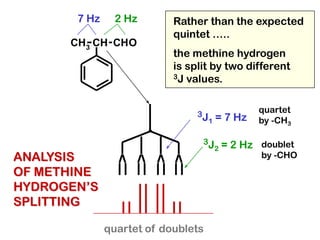
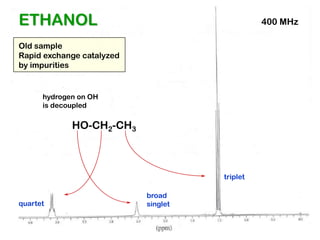
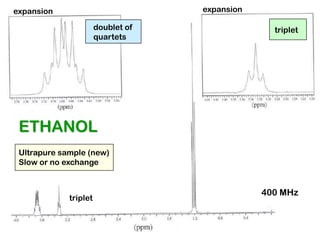
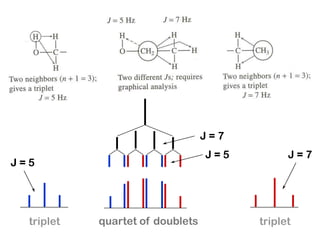
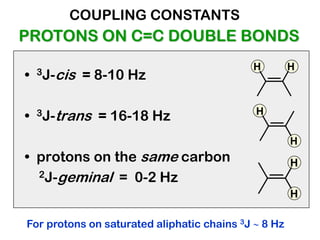
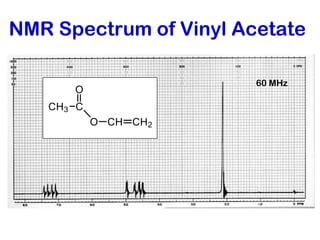
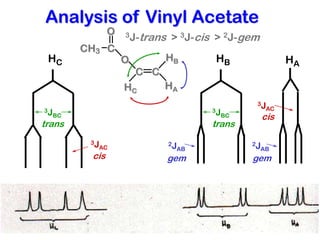
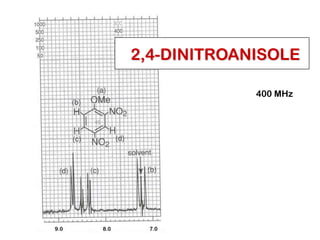
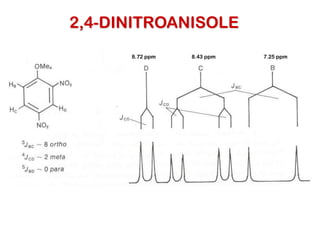
The document discusses the n+1 rule in NMR spectroscopy, detailing its application to tree diagrams for splitting patterns in aliphatic chains and rings. It highlights the conditions under which the n+1 rule is valid, primarily focusing on equal coupling constants and free rotation among hydrogens. Examples such as 2-phenylpropanal and vinyl acetate further illustrate how unequal j values affect splitting patterns and NMR analysis.