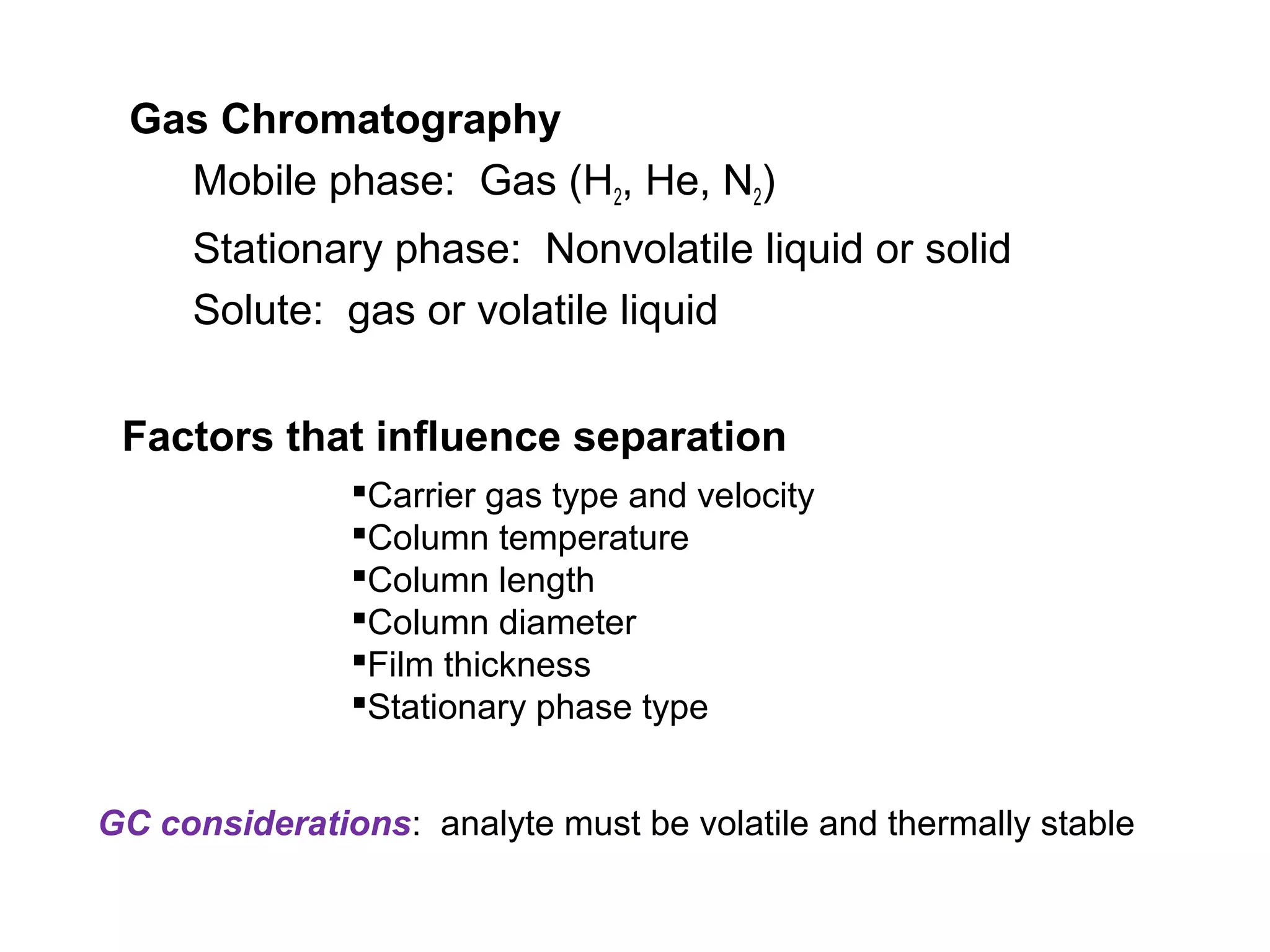
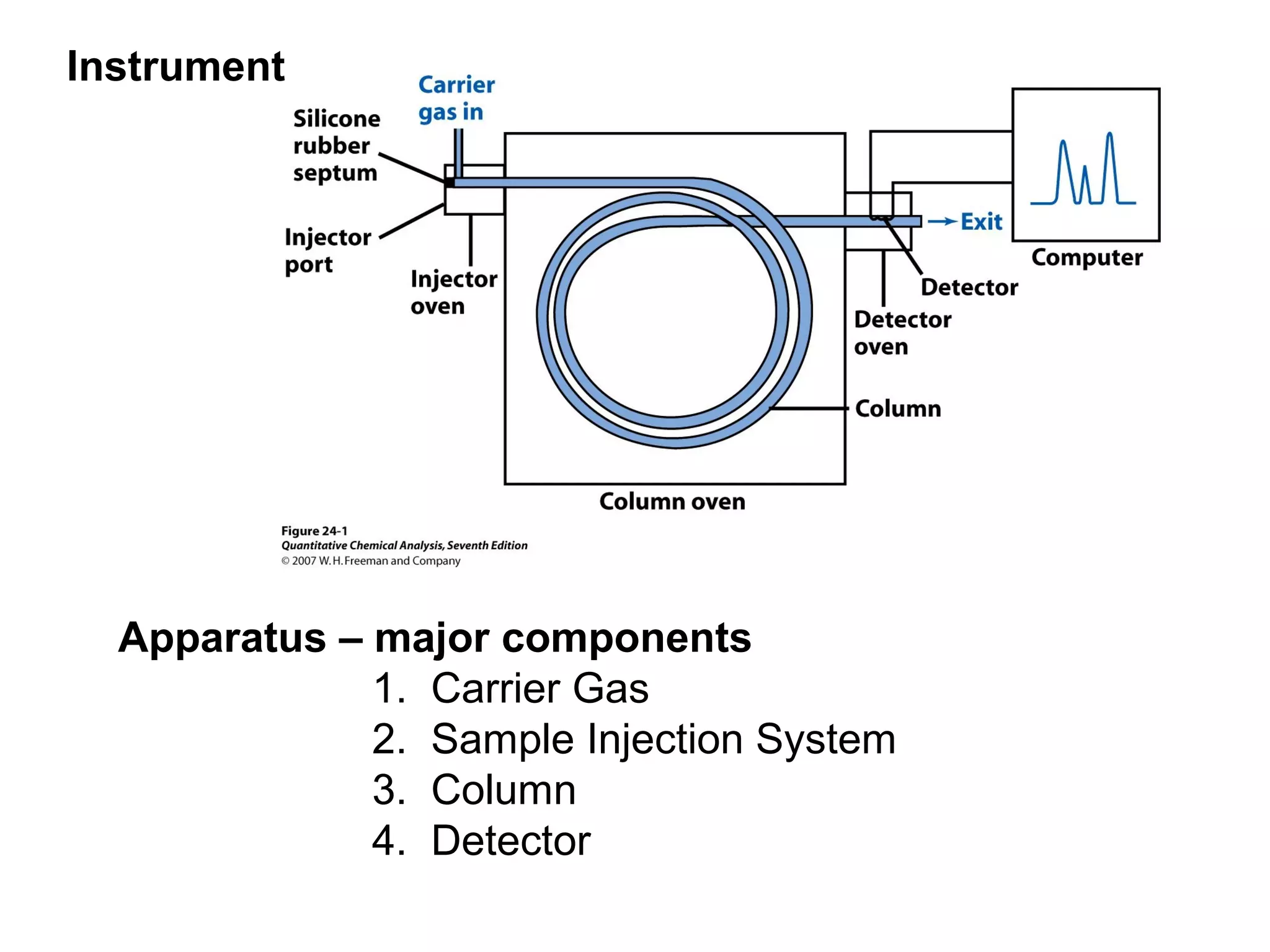
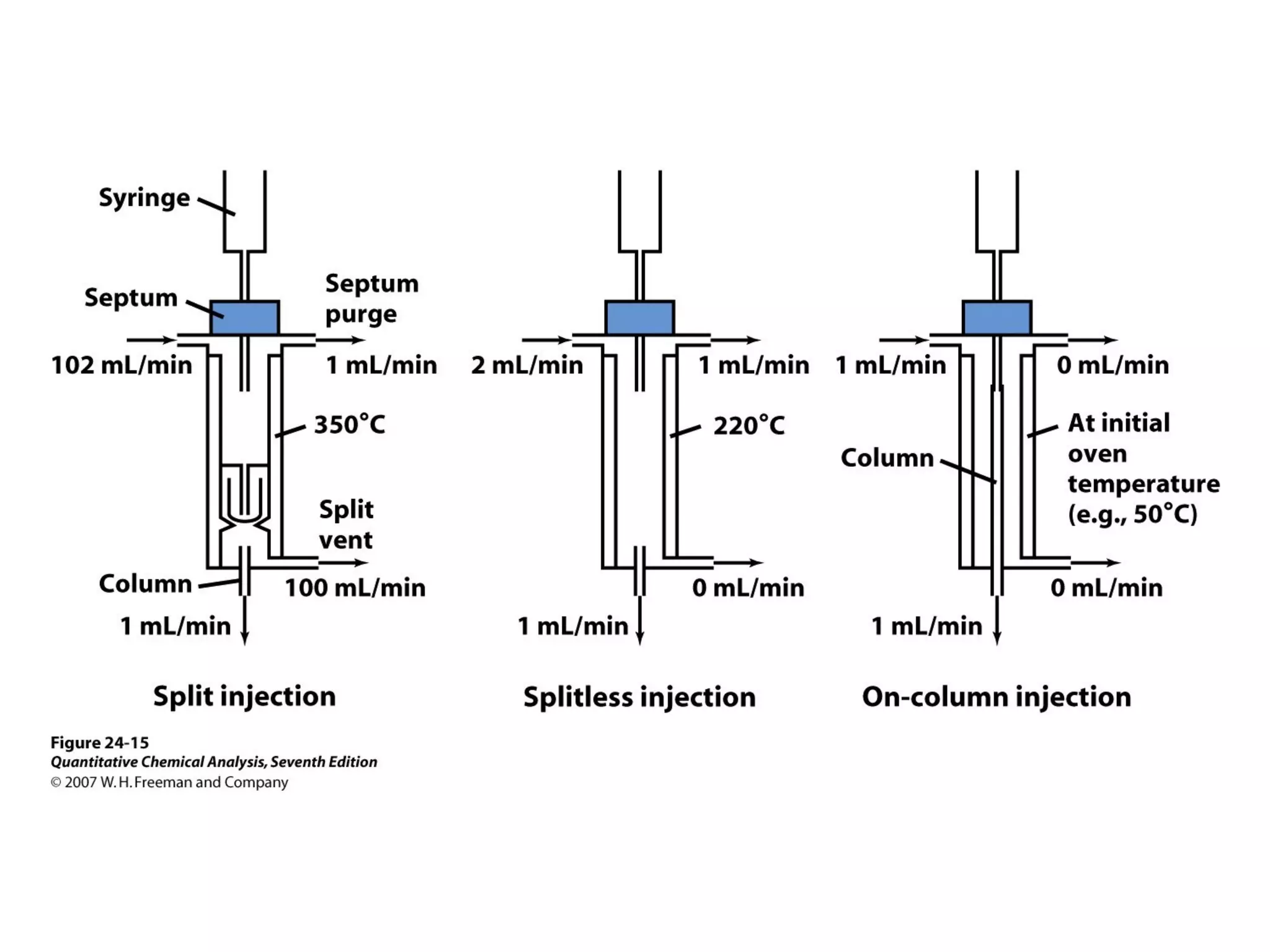
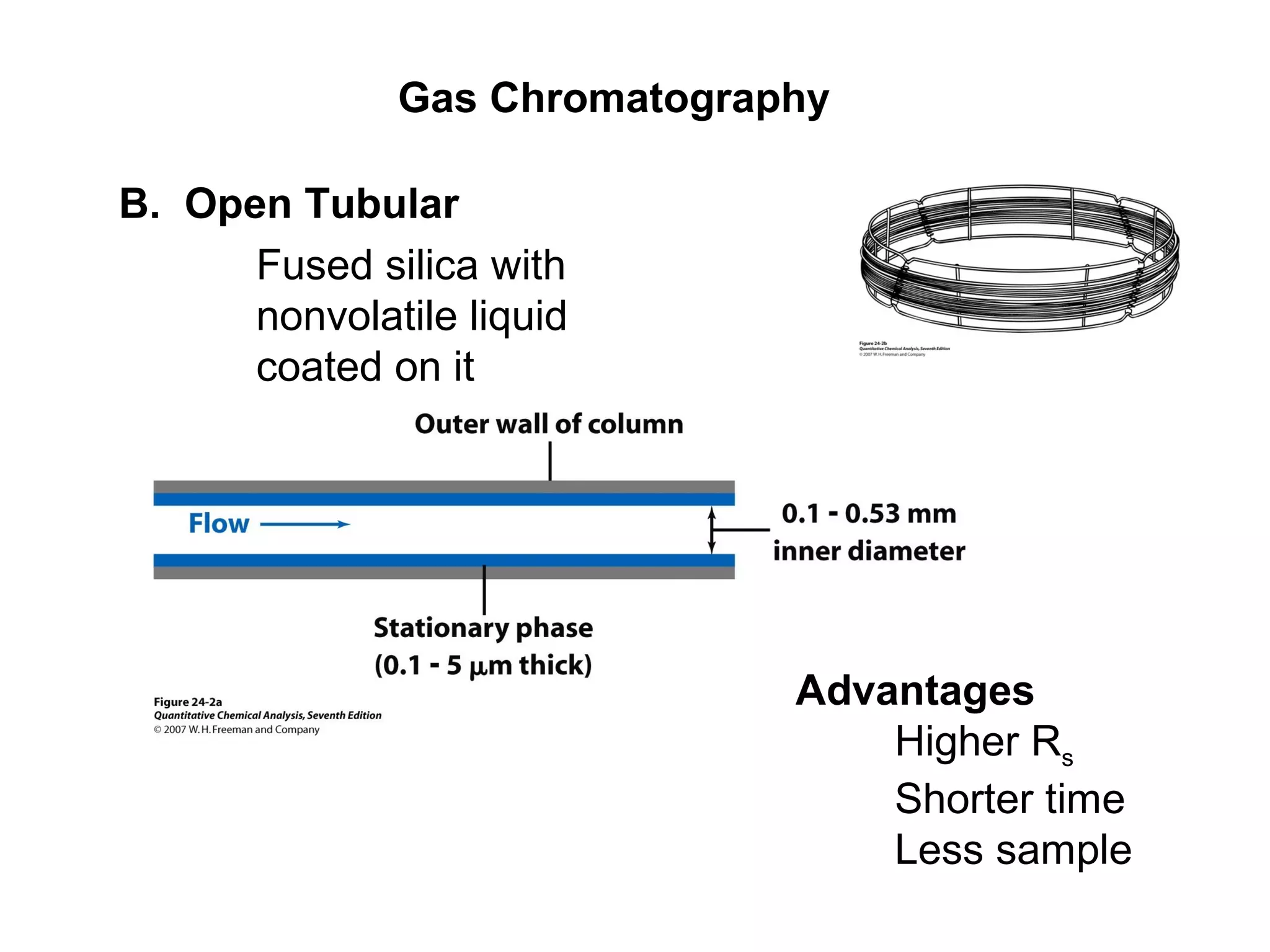
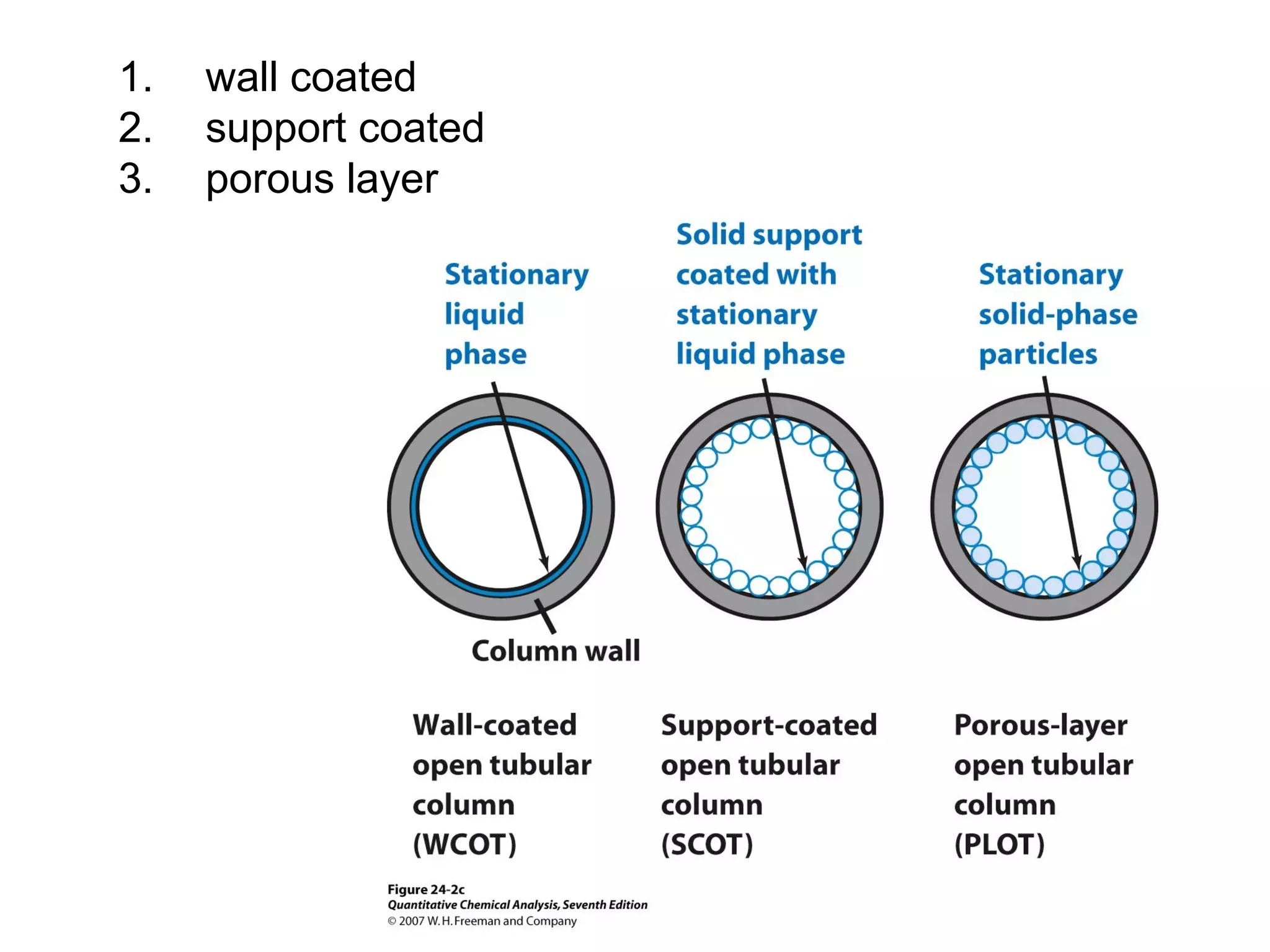
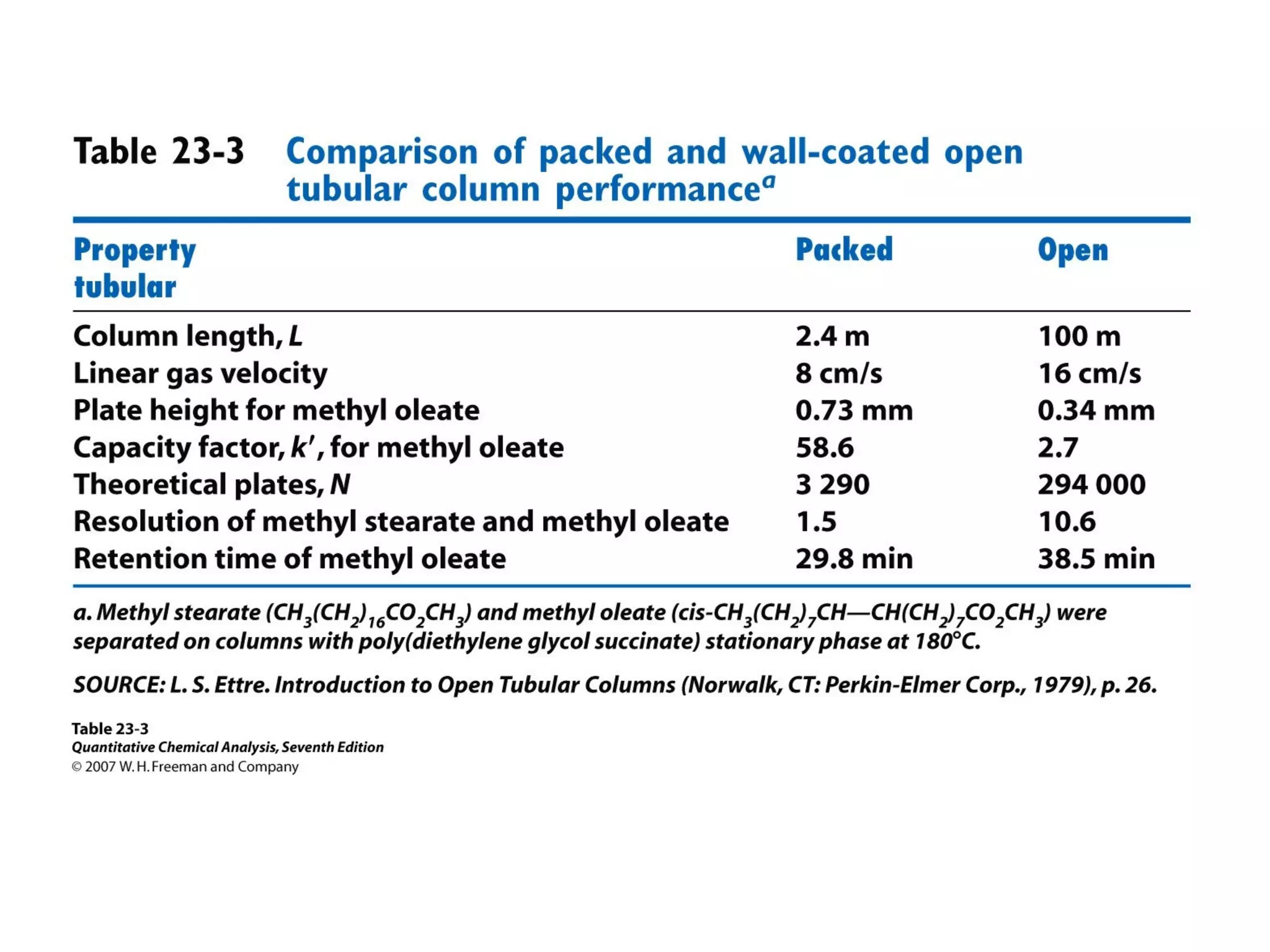
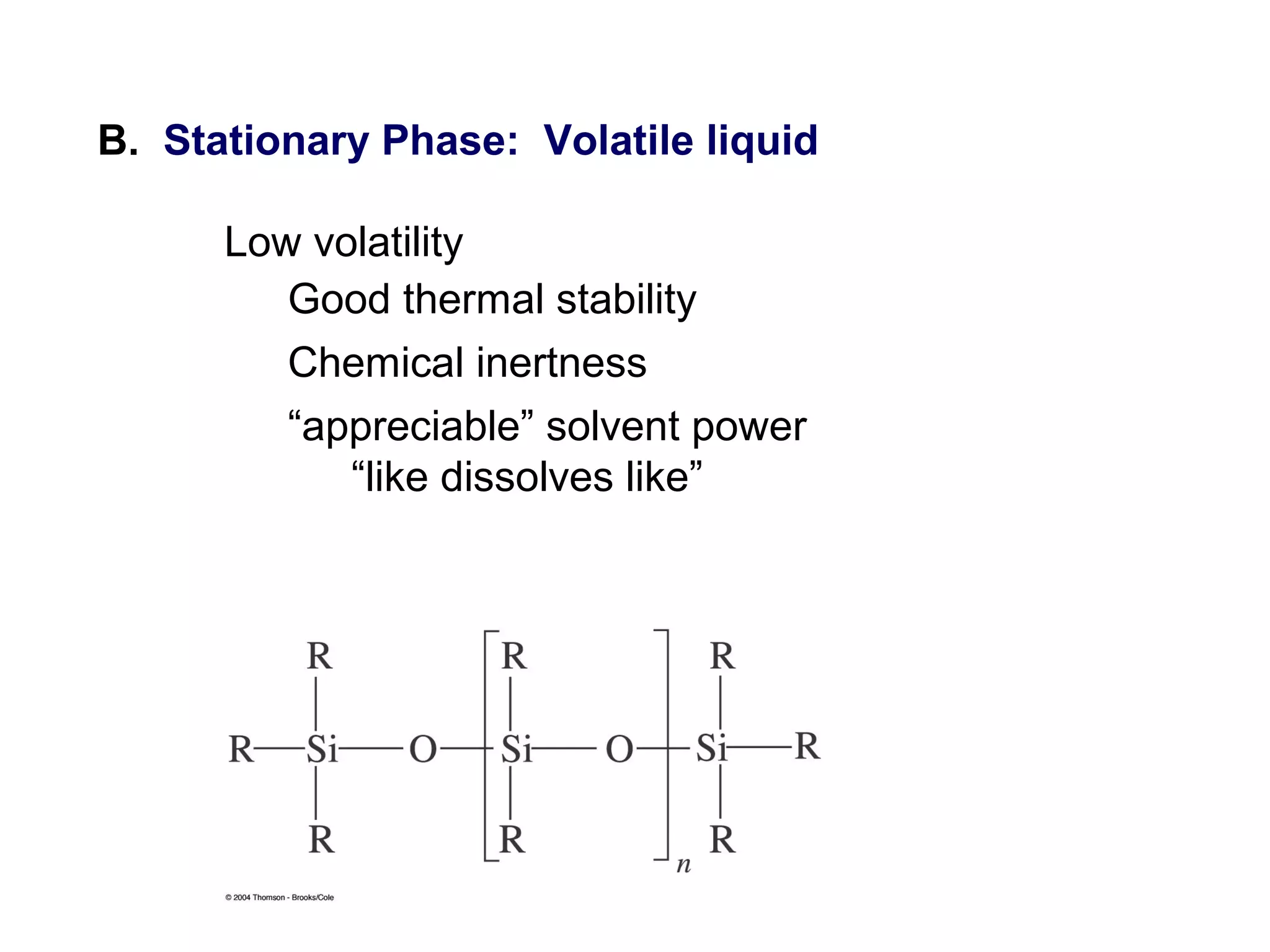
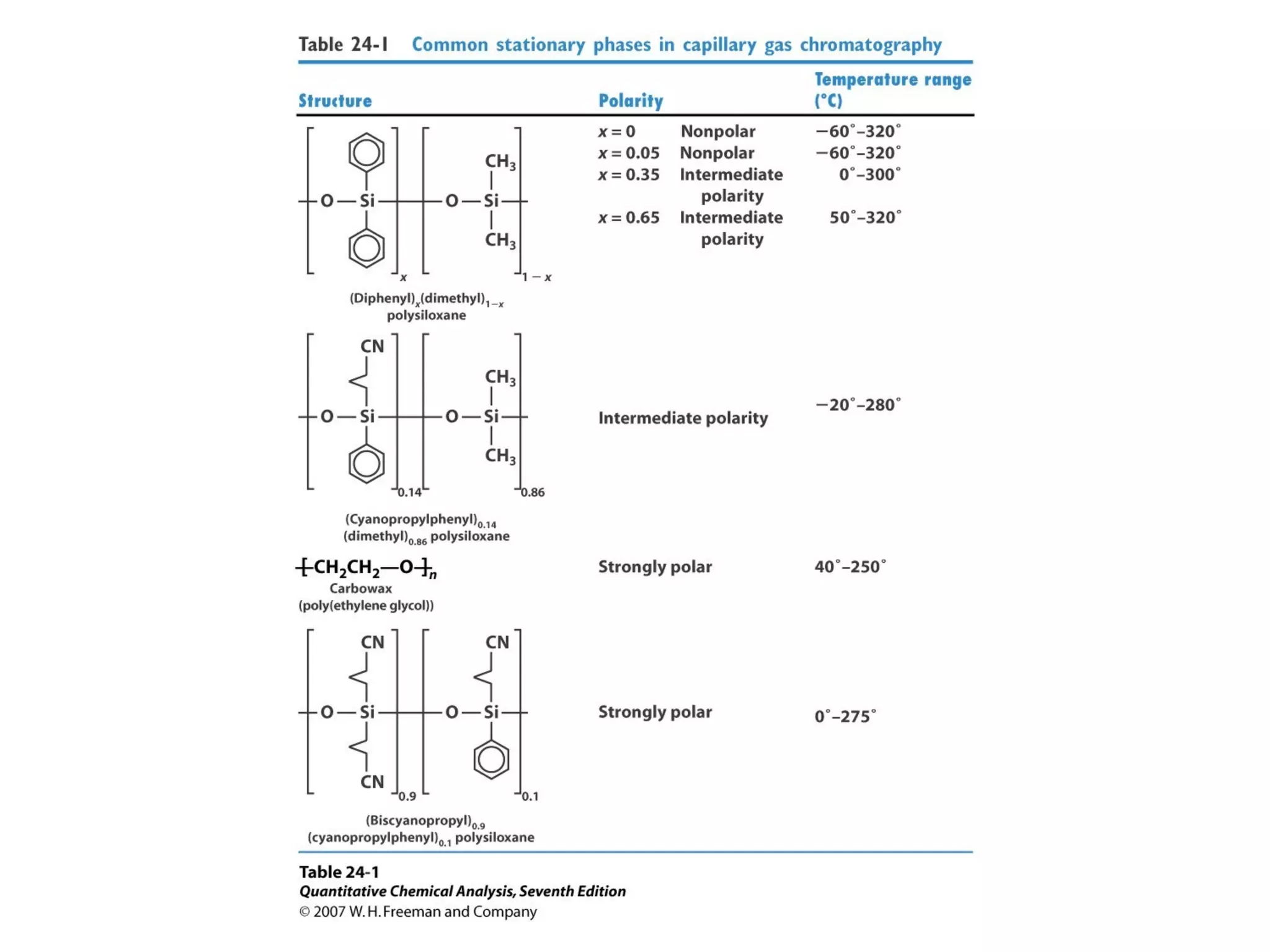
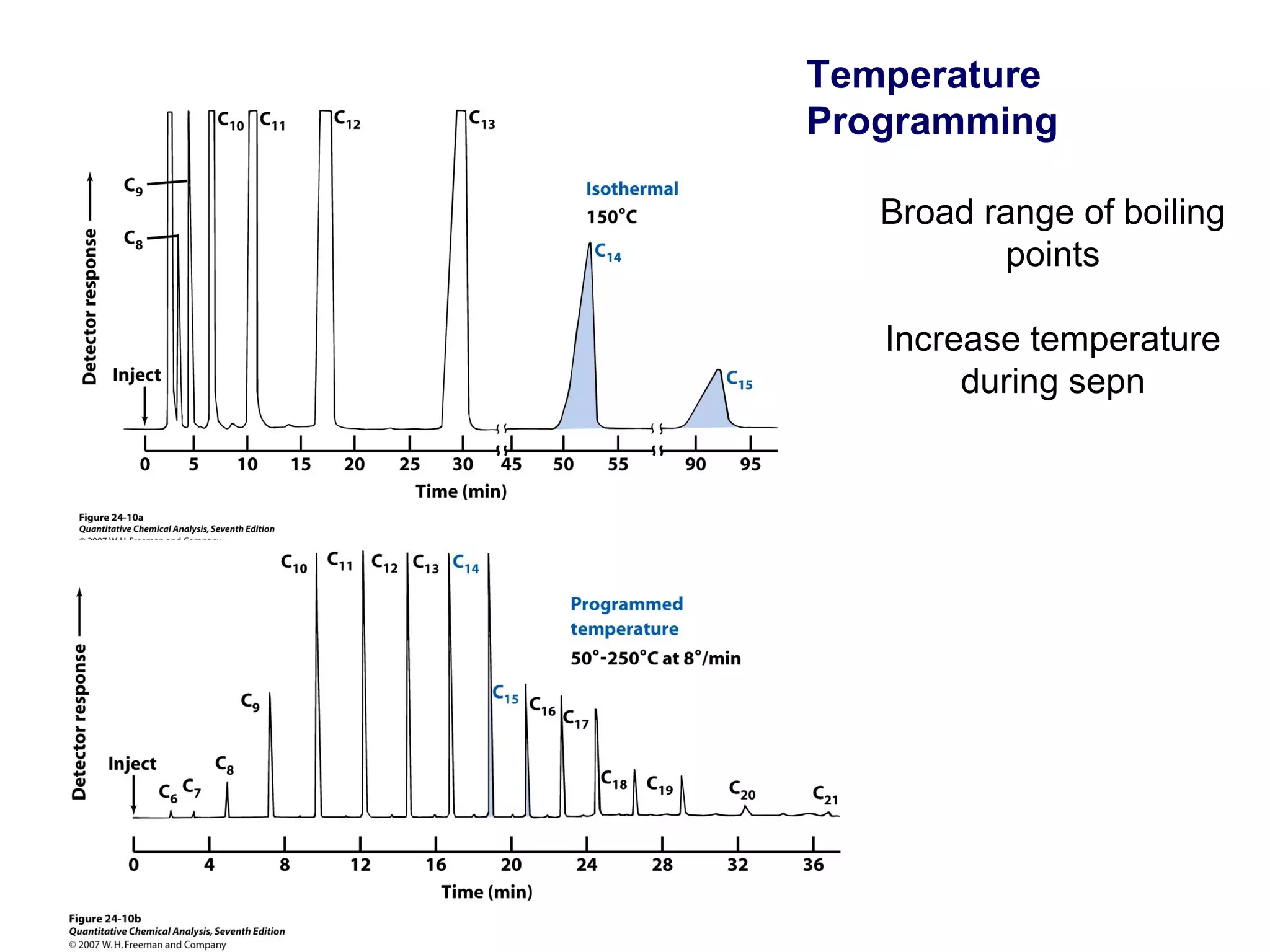
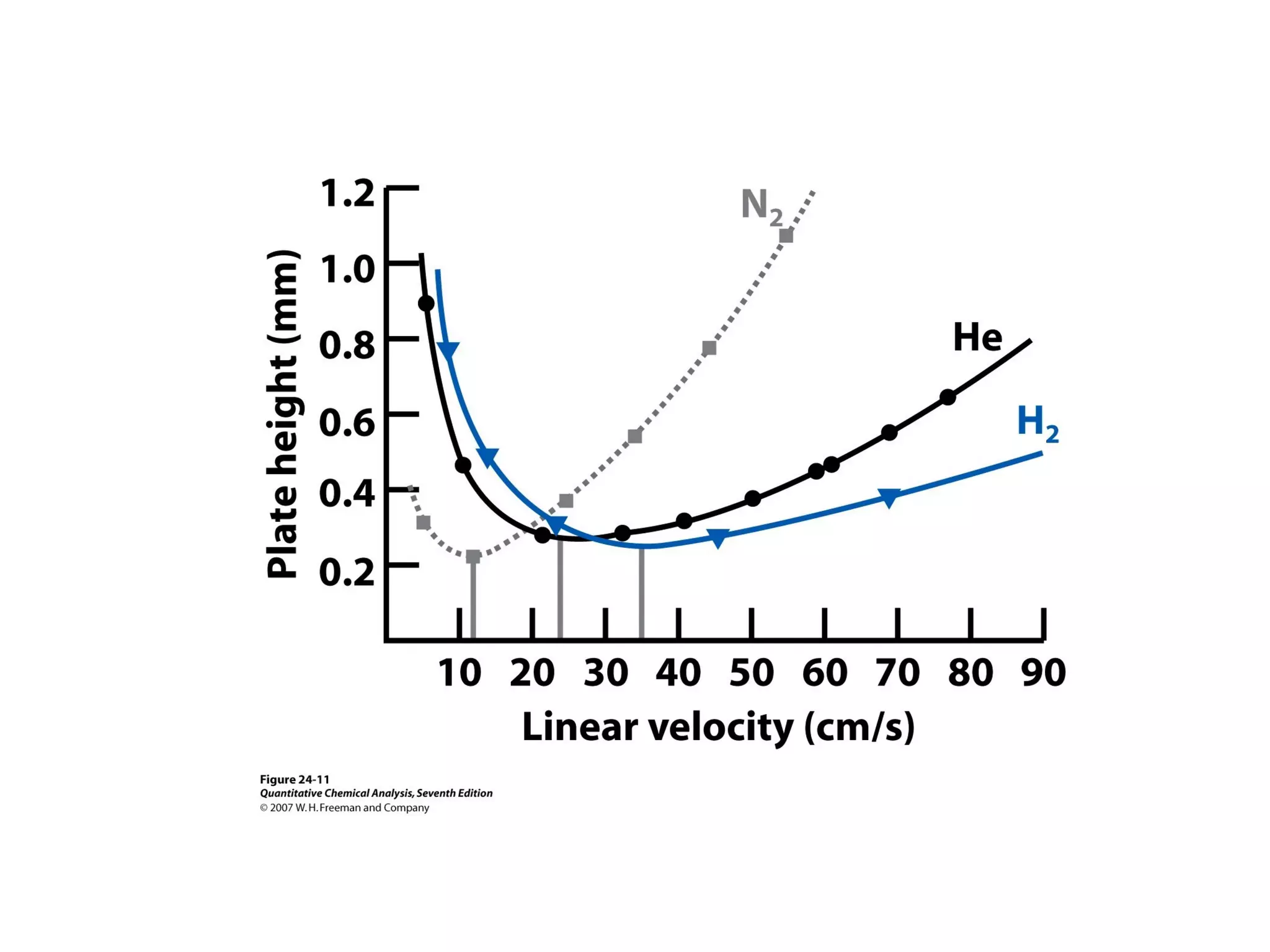
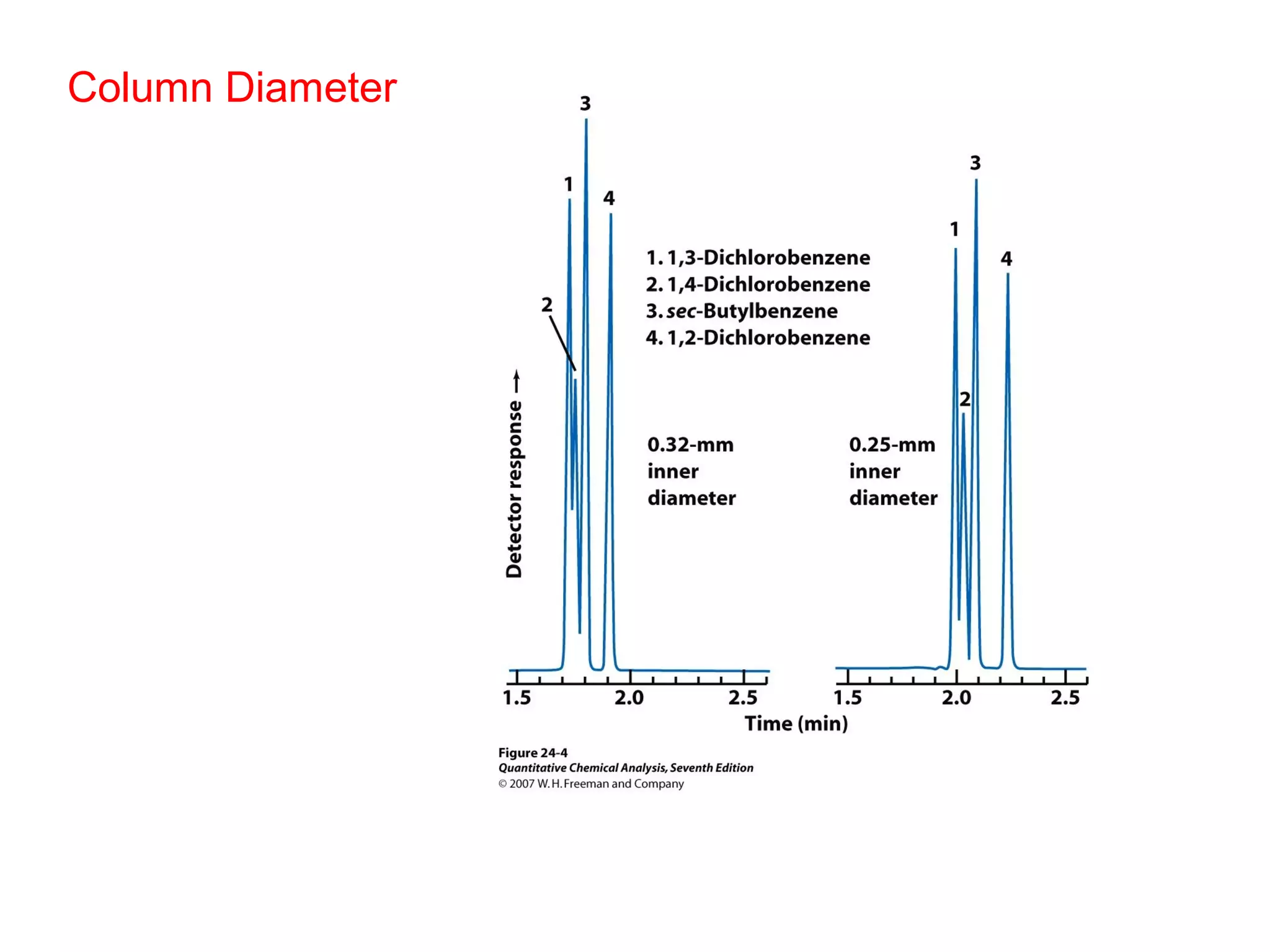
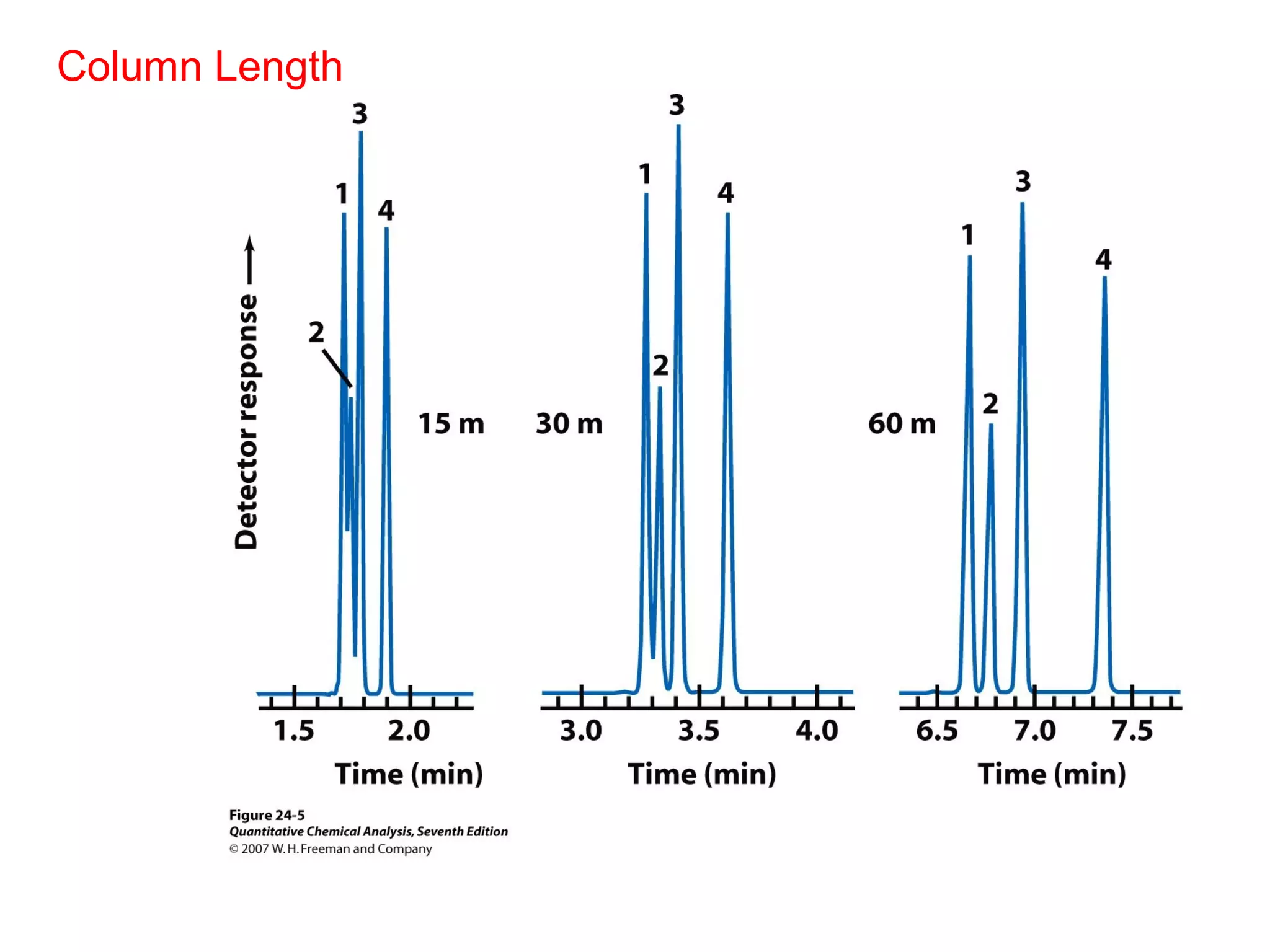
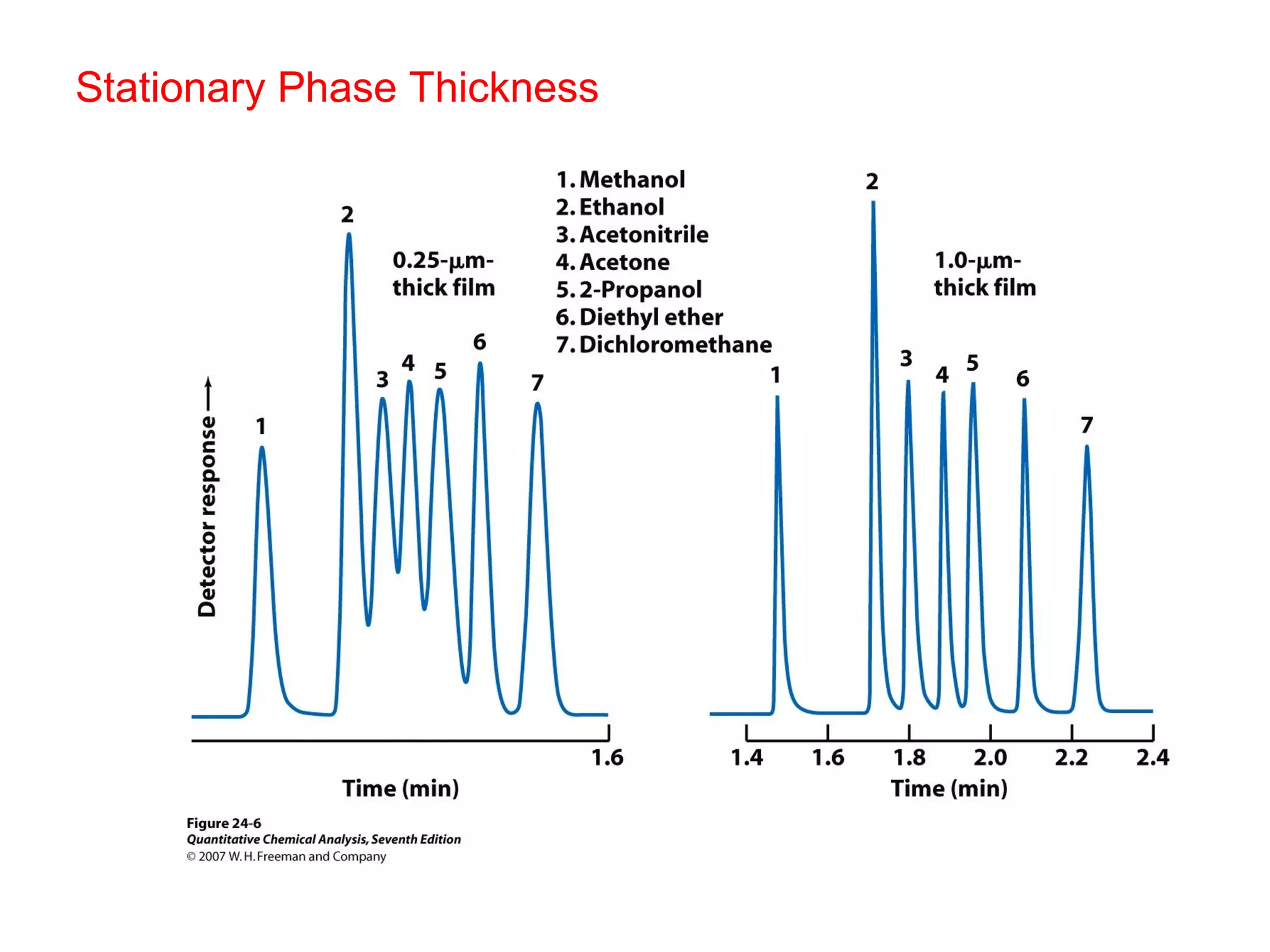
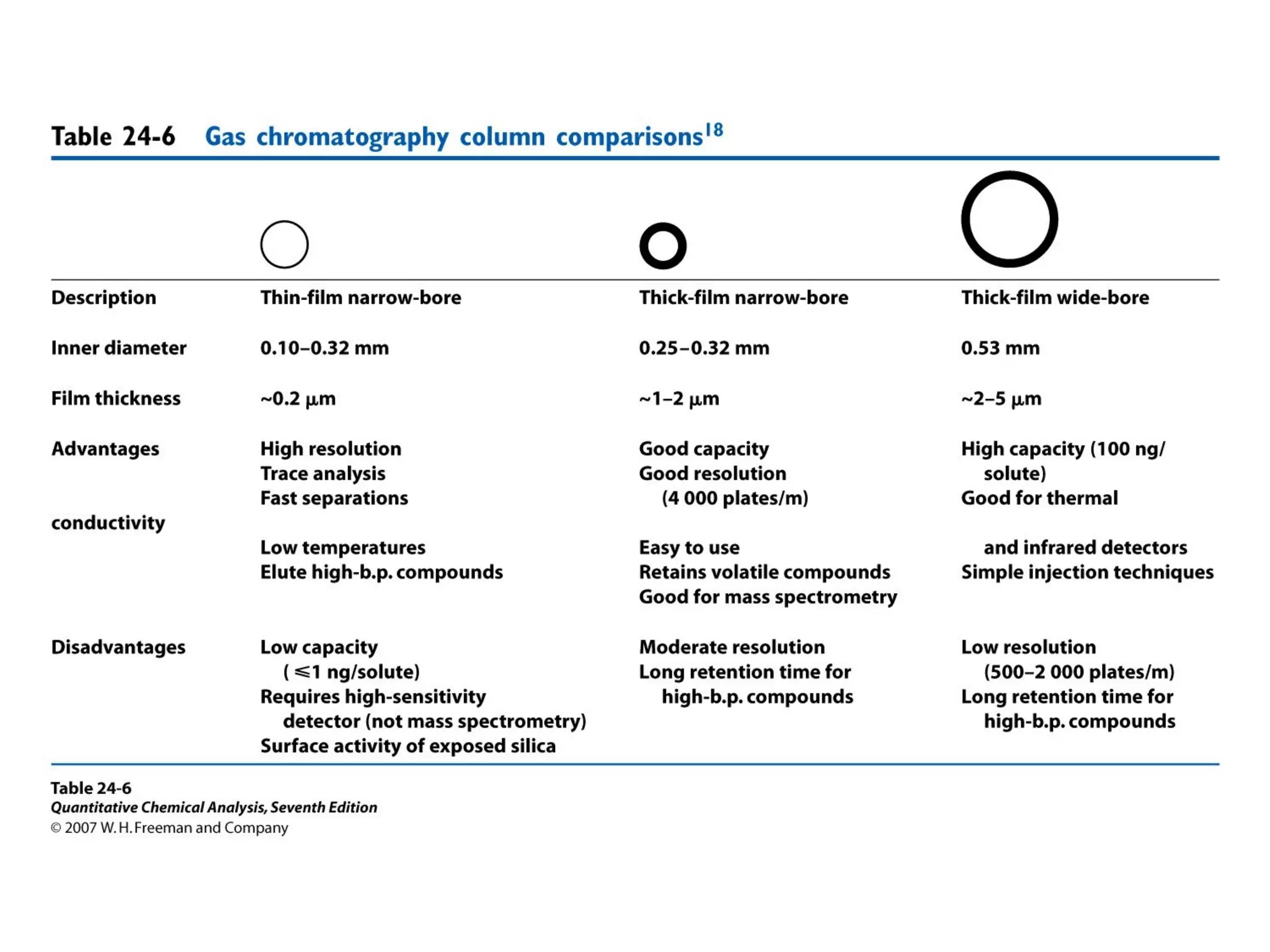
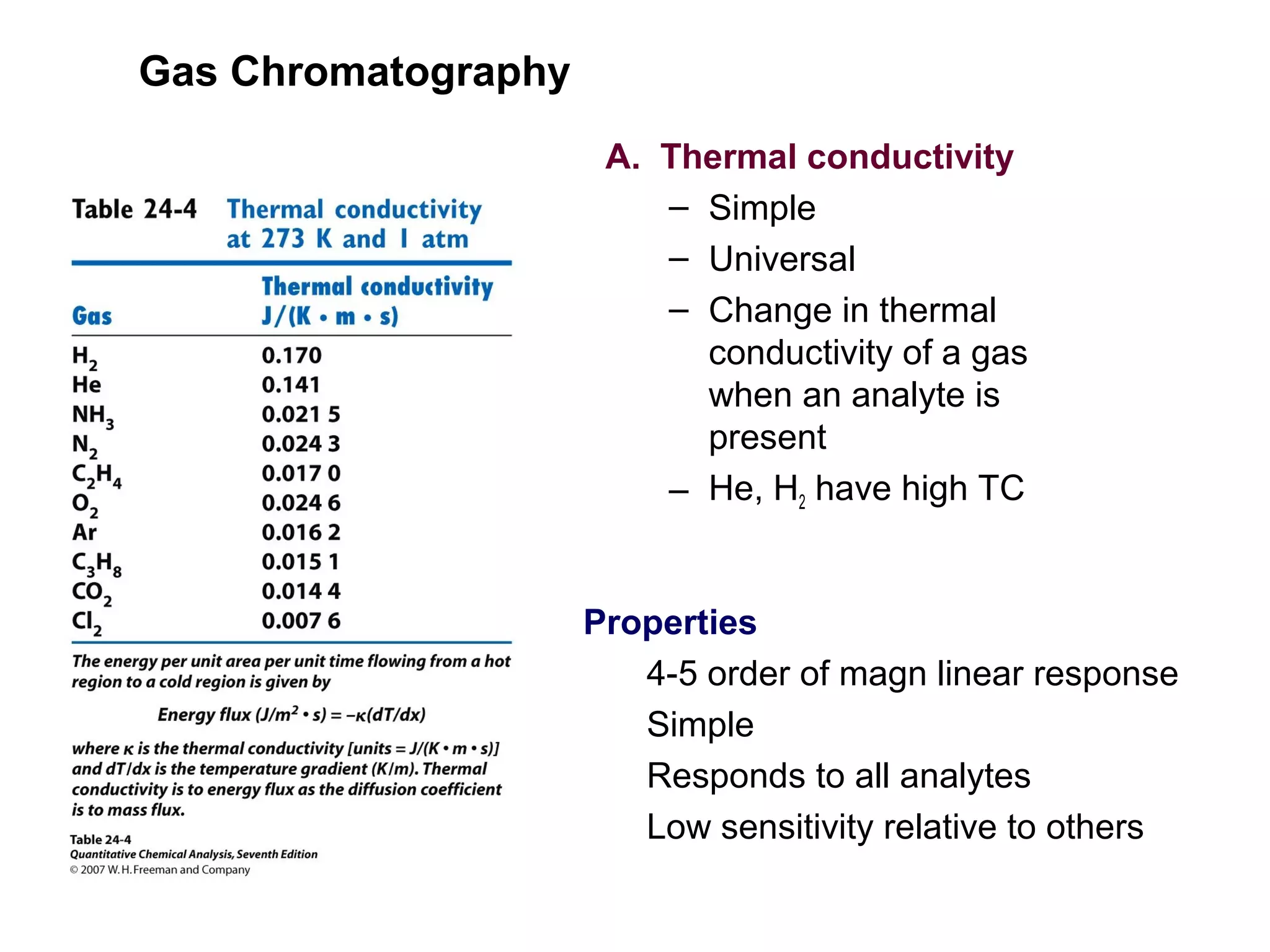
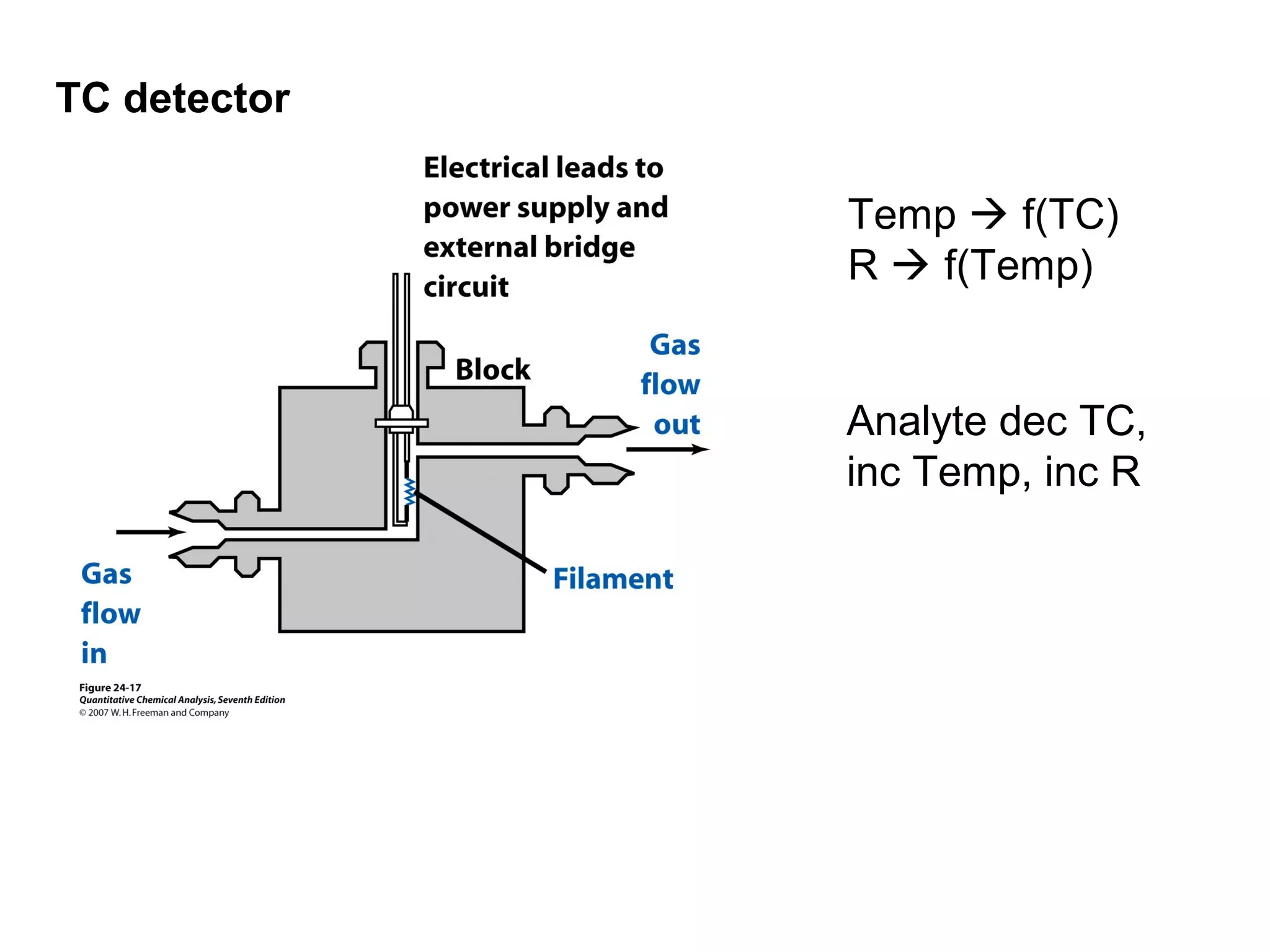
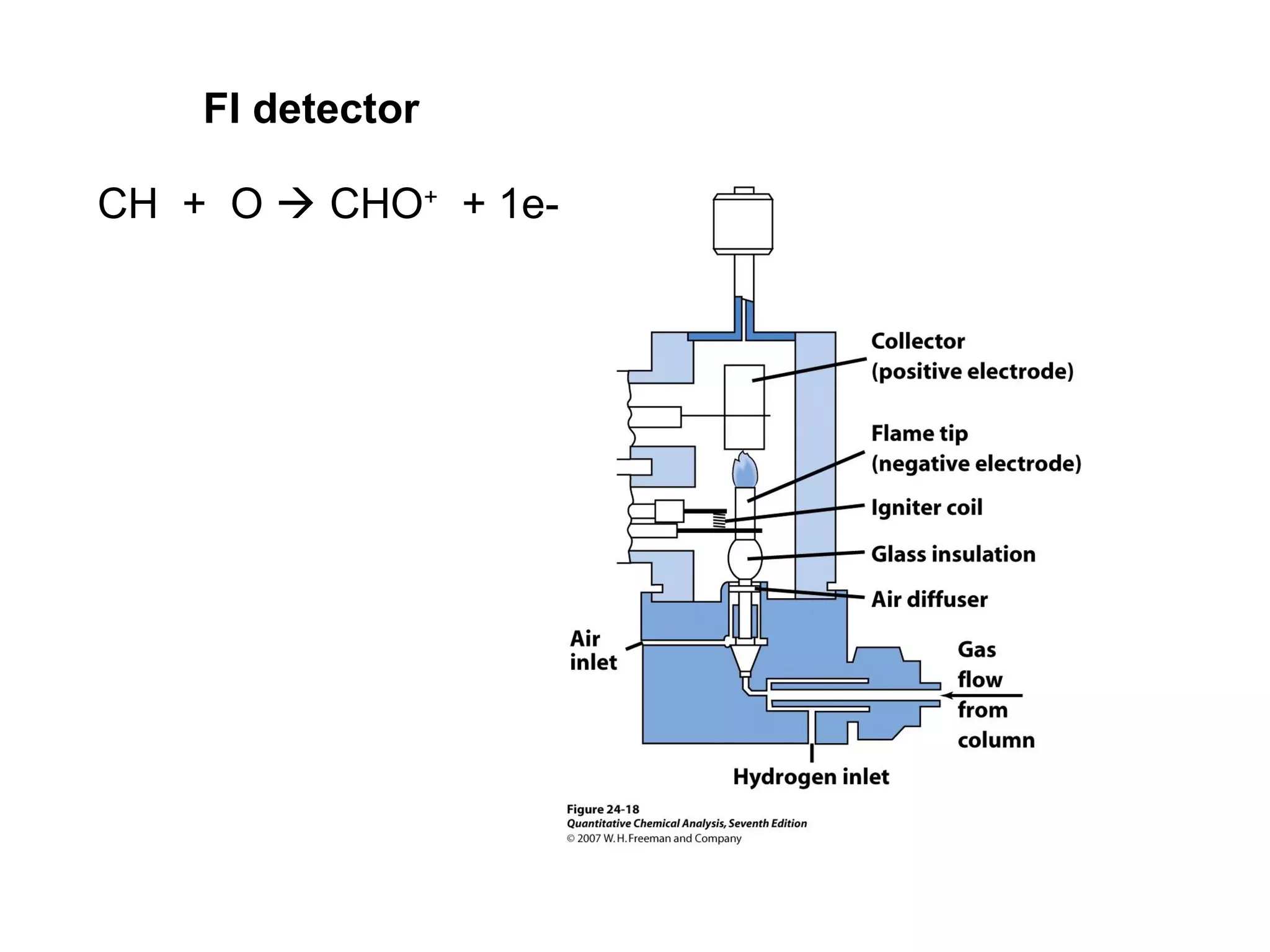
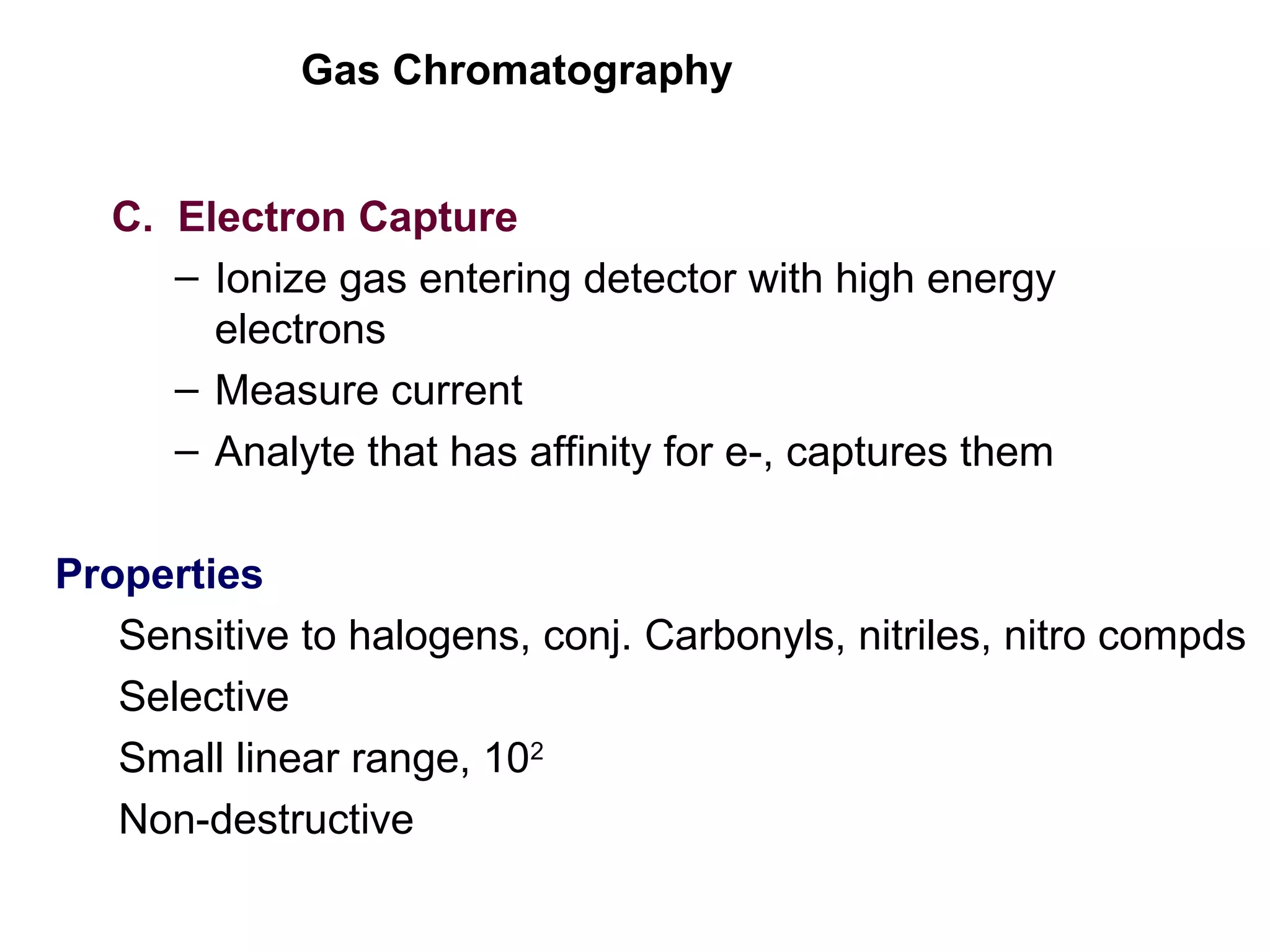
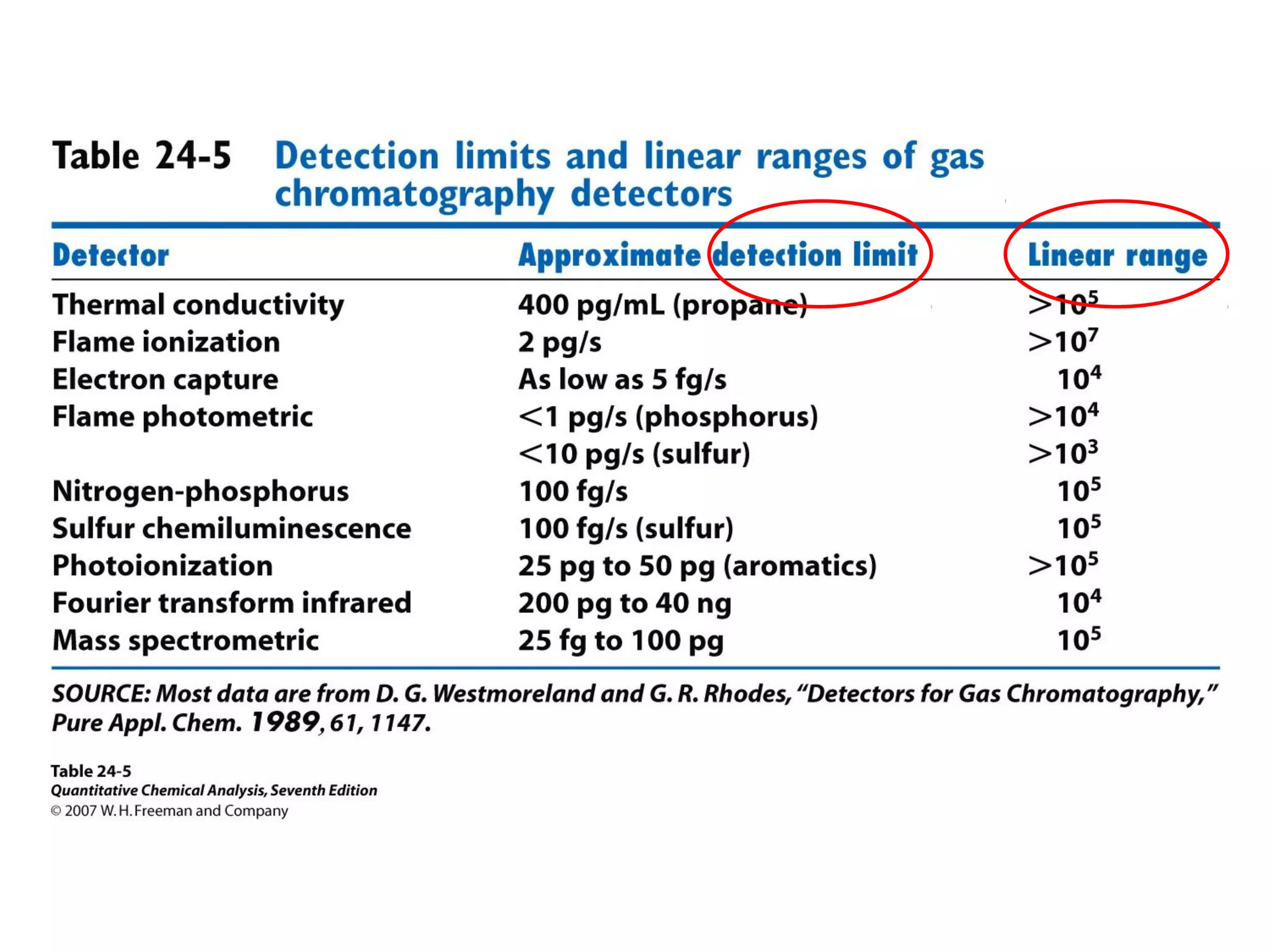
Gas chromatography is a technique used to separate and analyze mixtures that rely on differences in volatility and affinity of compounds for a mobile and stationary phase. The key components of a gas chromatography system are a carrier gas, sample injection system, column, and detector. Factors like carrier gas type, column temperature, length, diameter, and stationary phase influence separation of compounds on the column. Common detectors include thermal conductivity, flame ionization, and electron capture detectors which have different properties in terms of sensitivity, selectivity, and response characteristics.