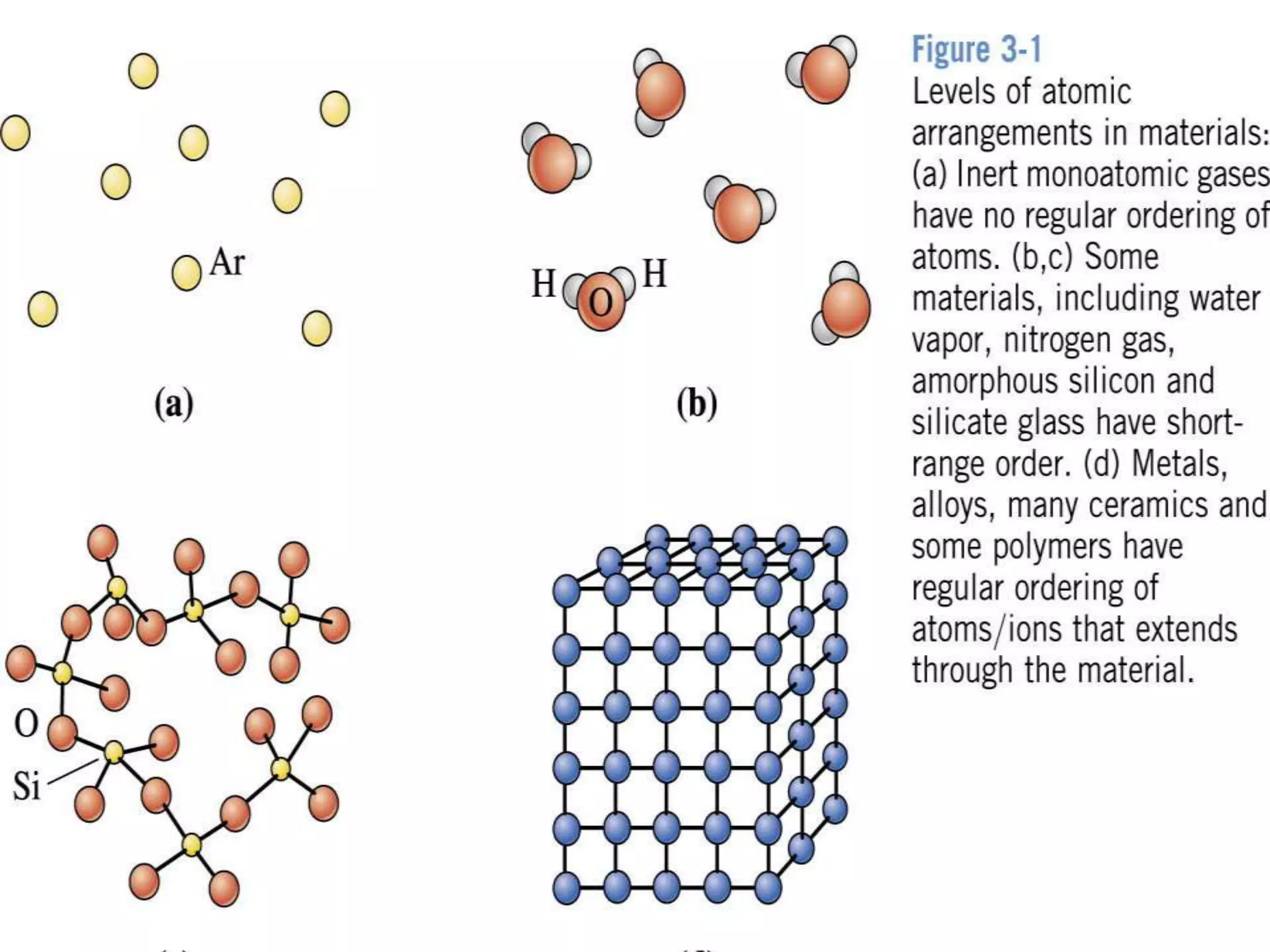
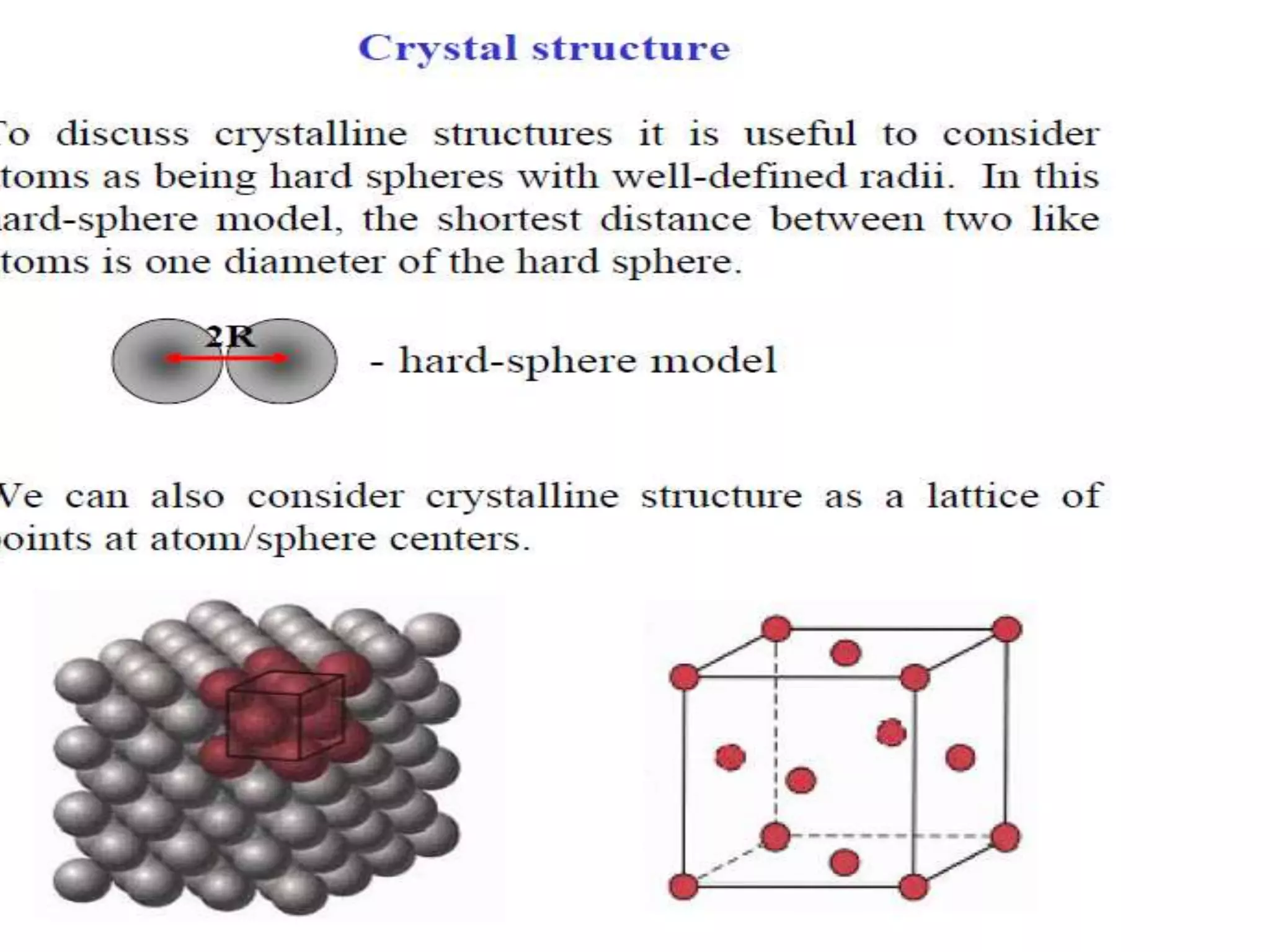
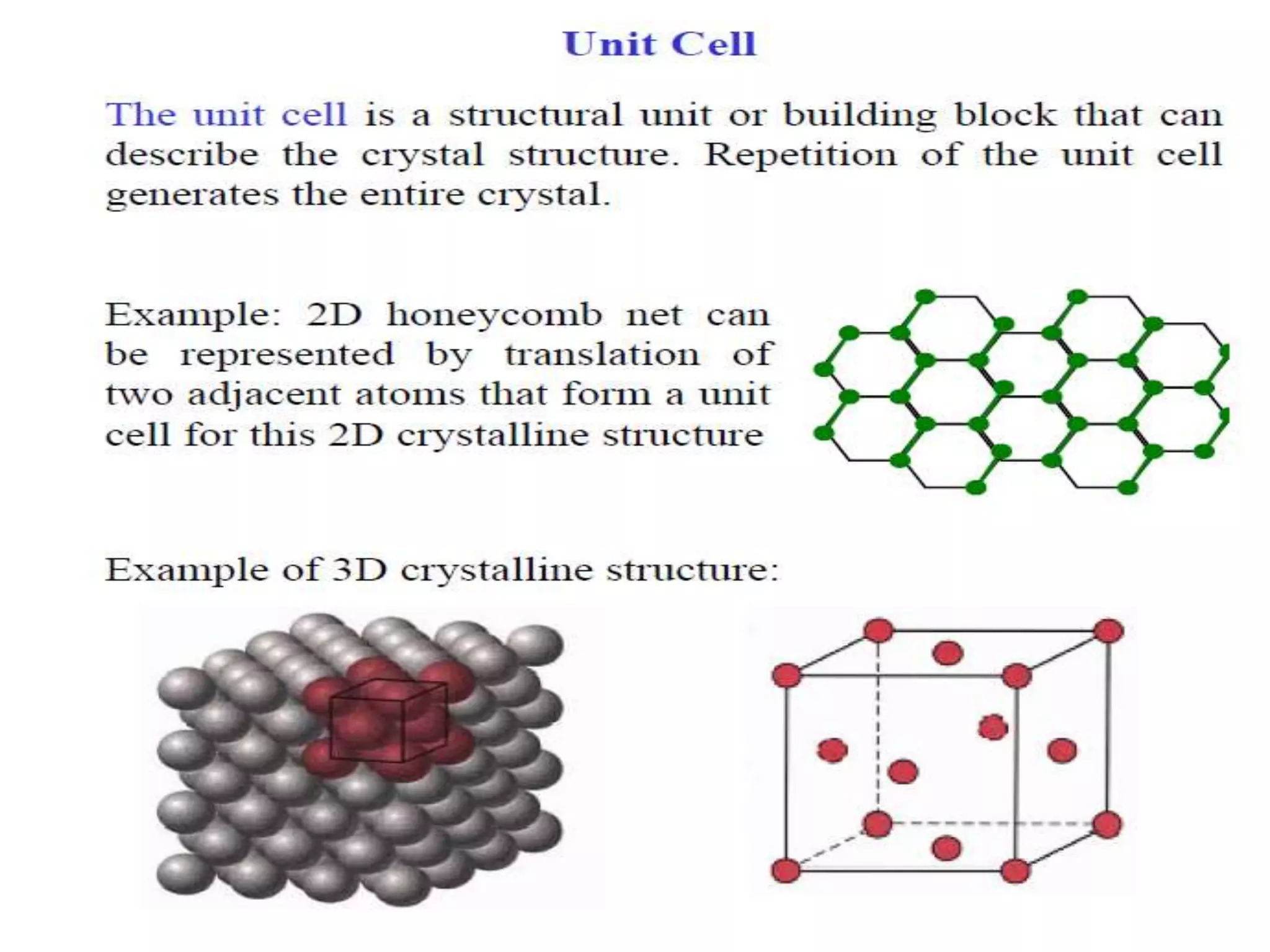
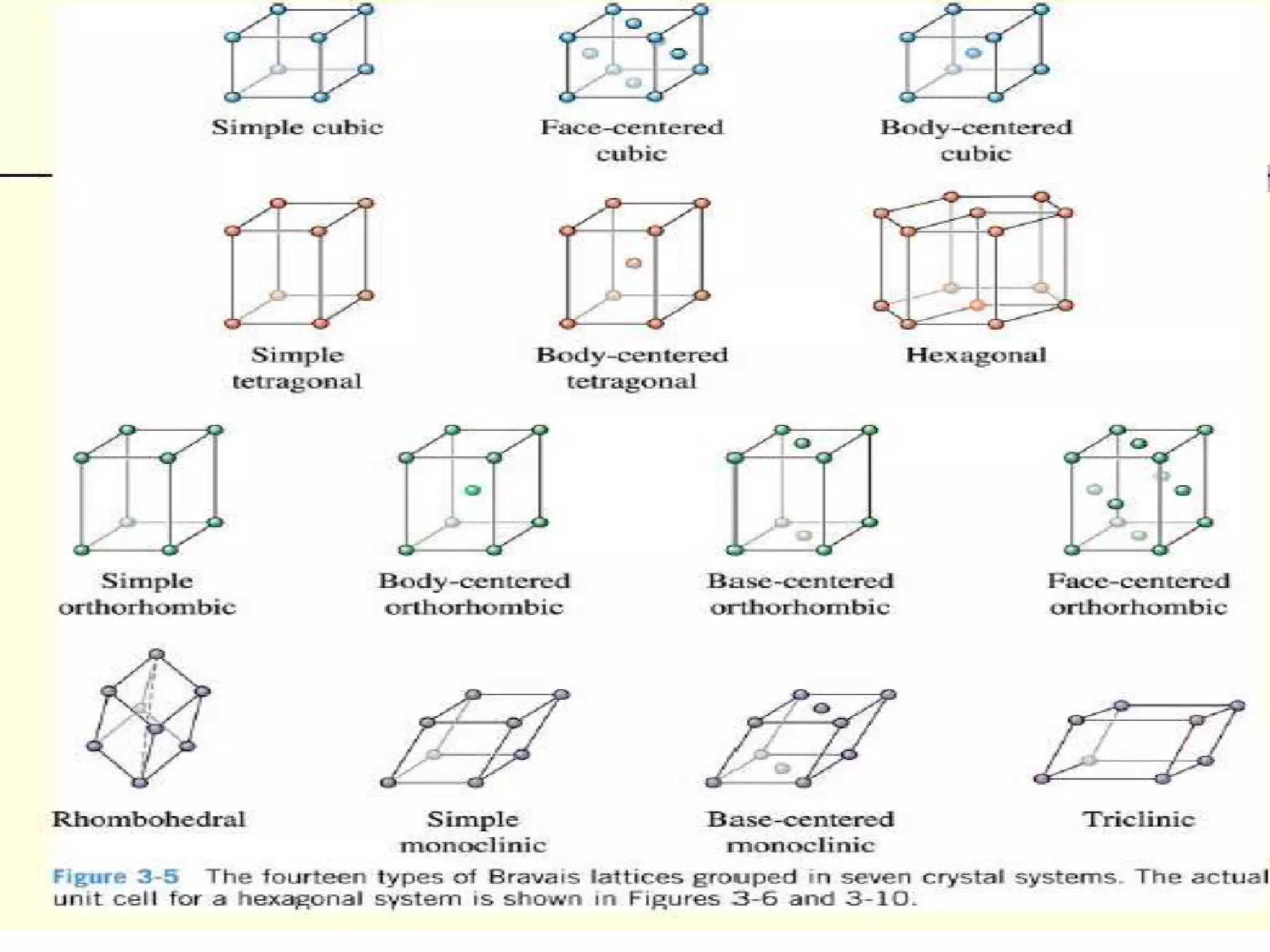
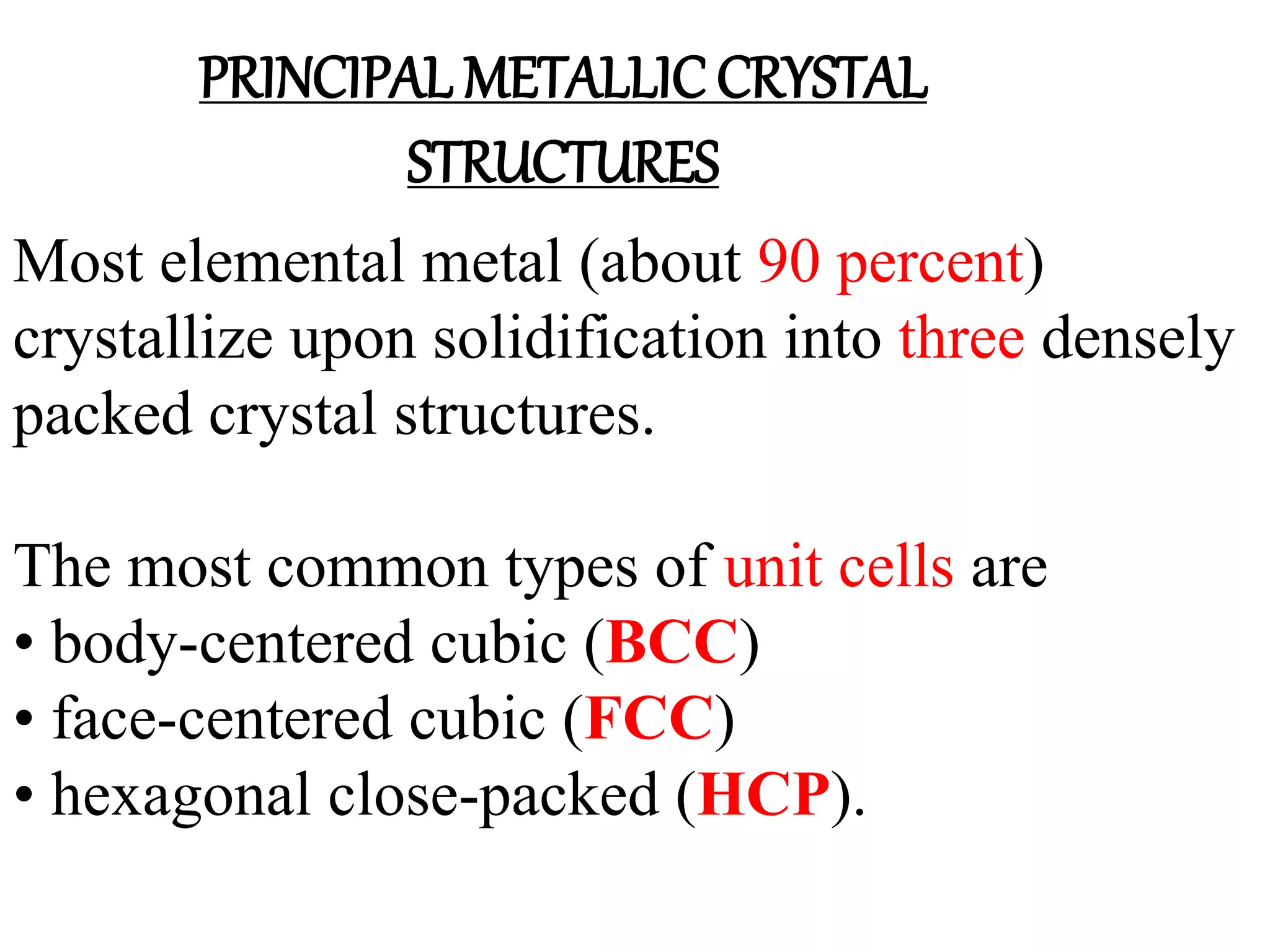
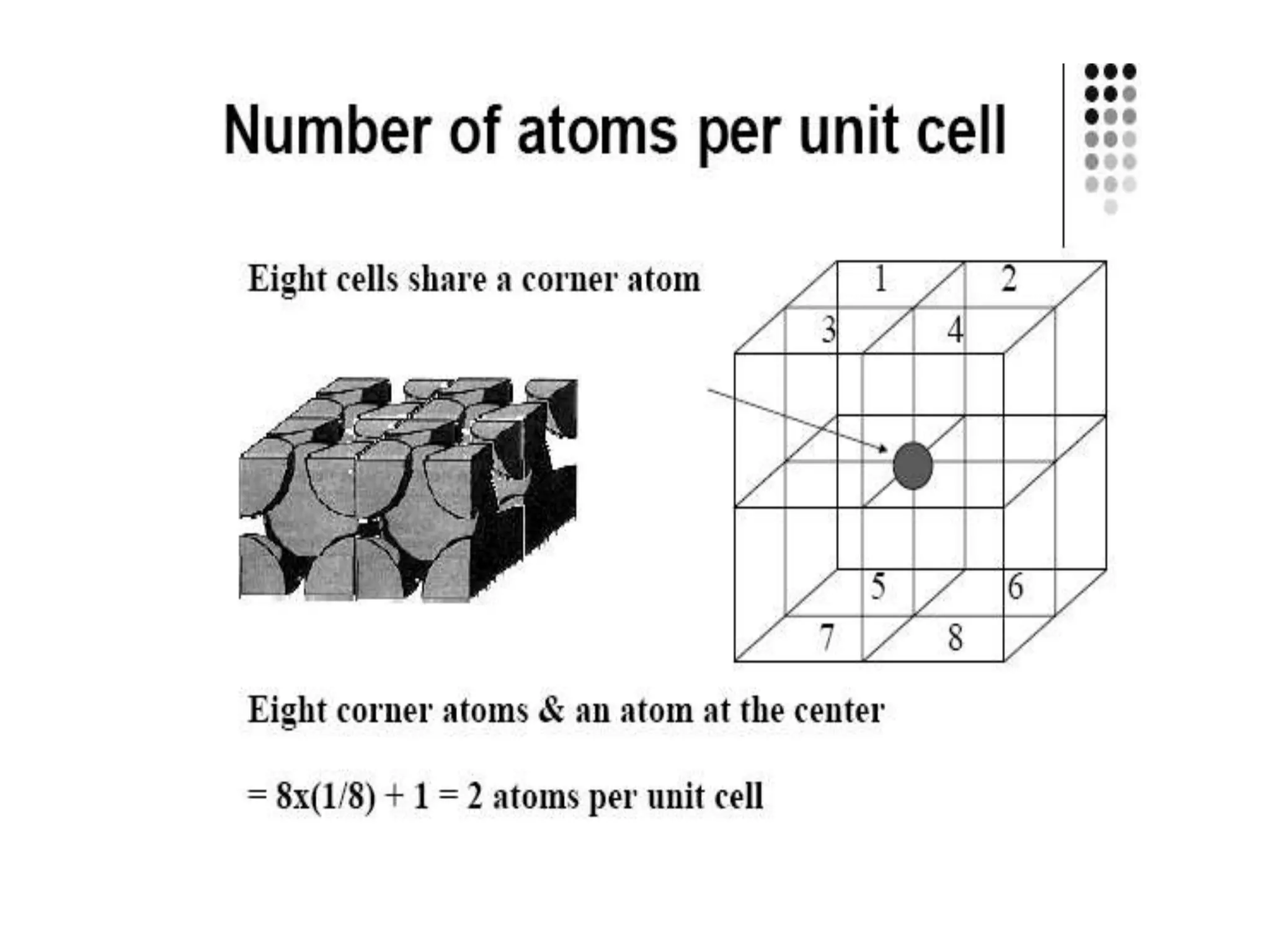
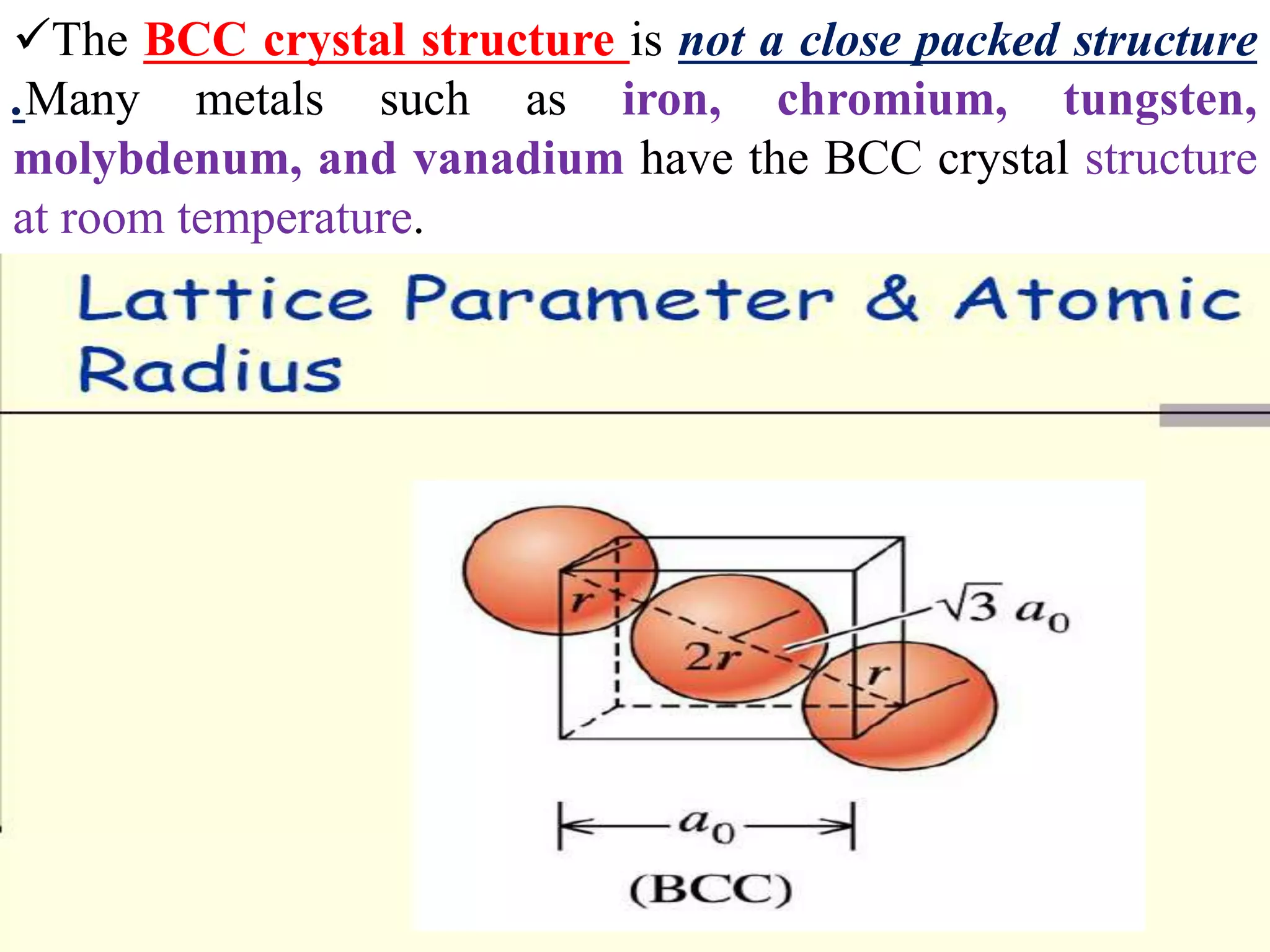
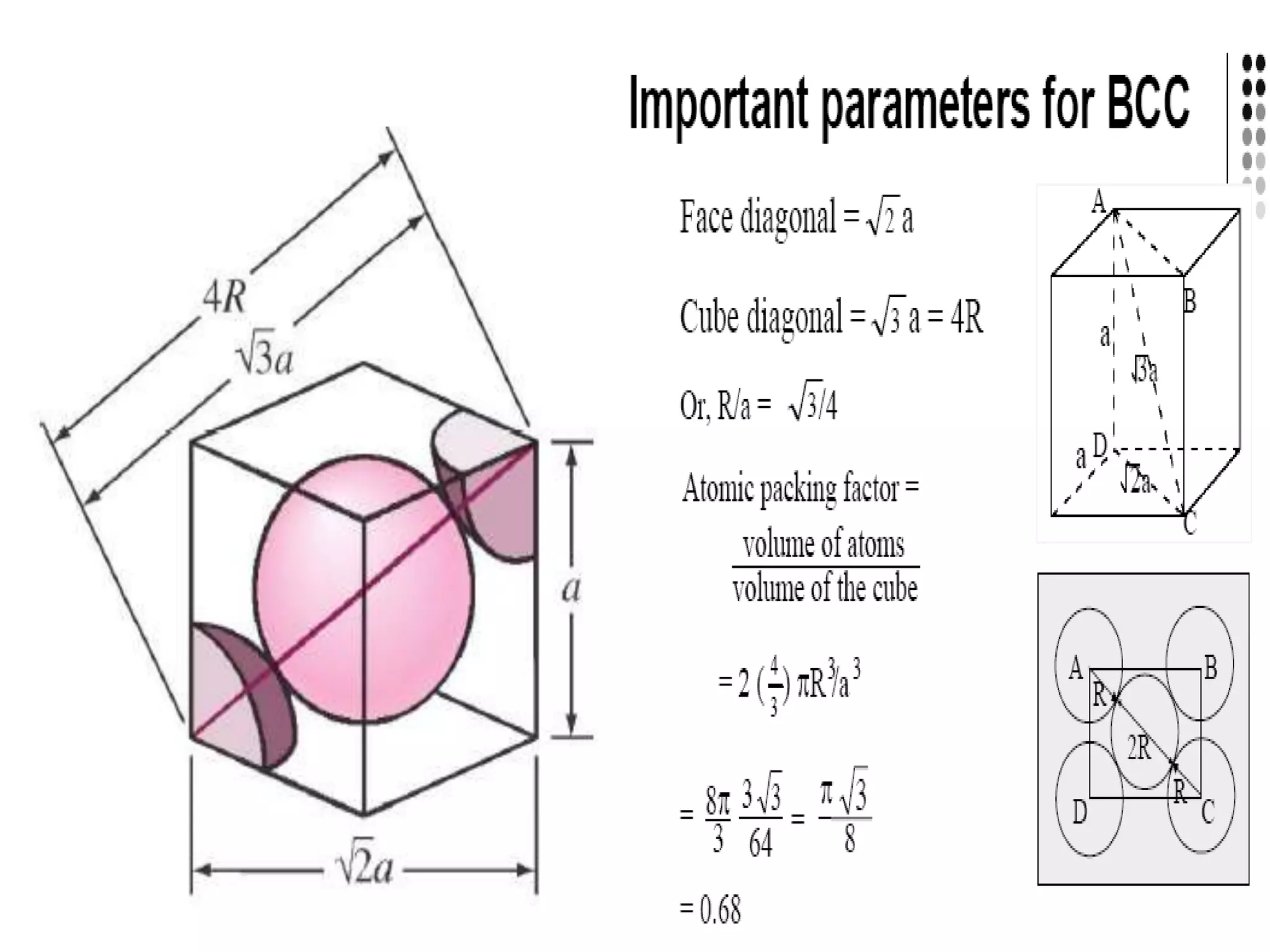
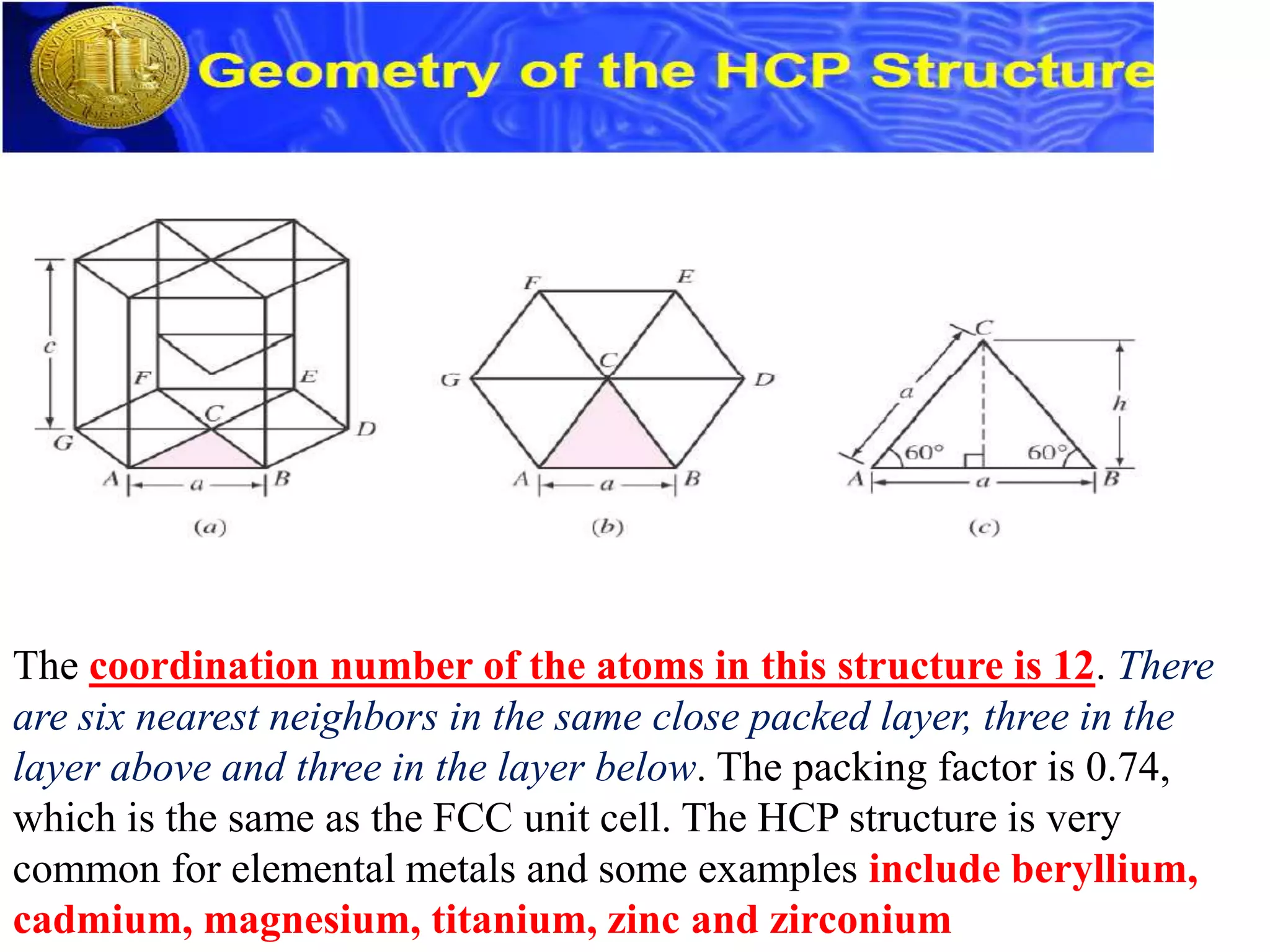
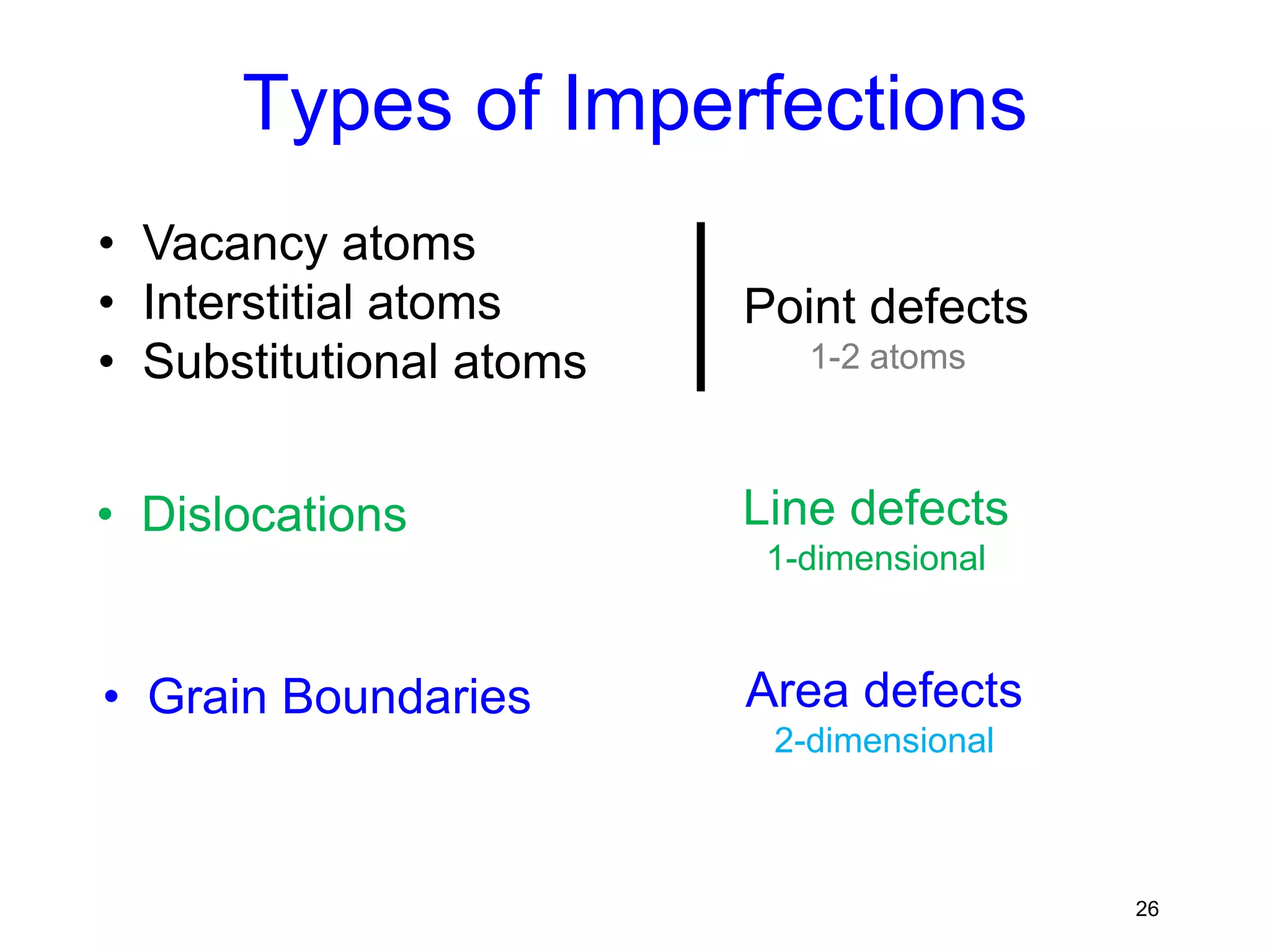
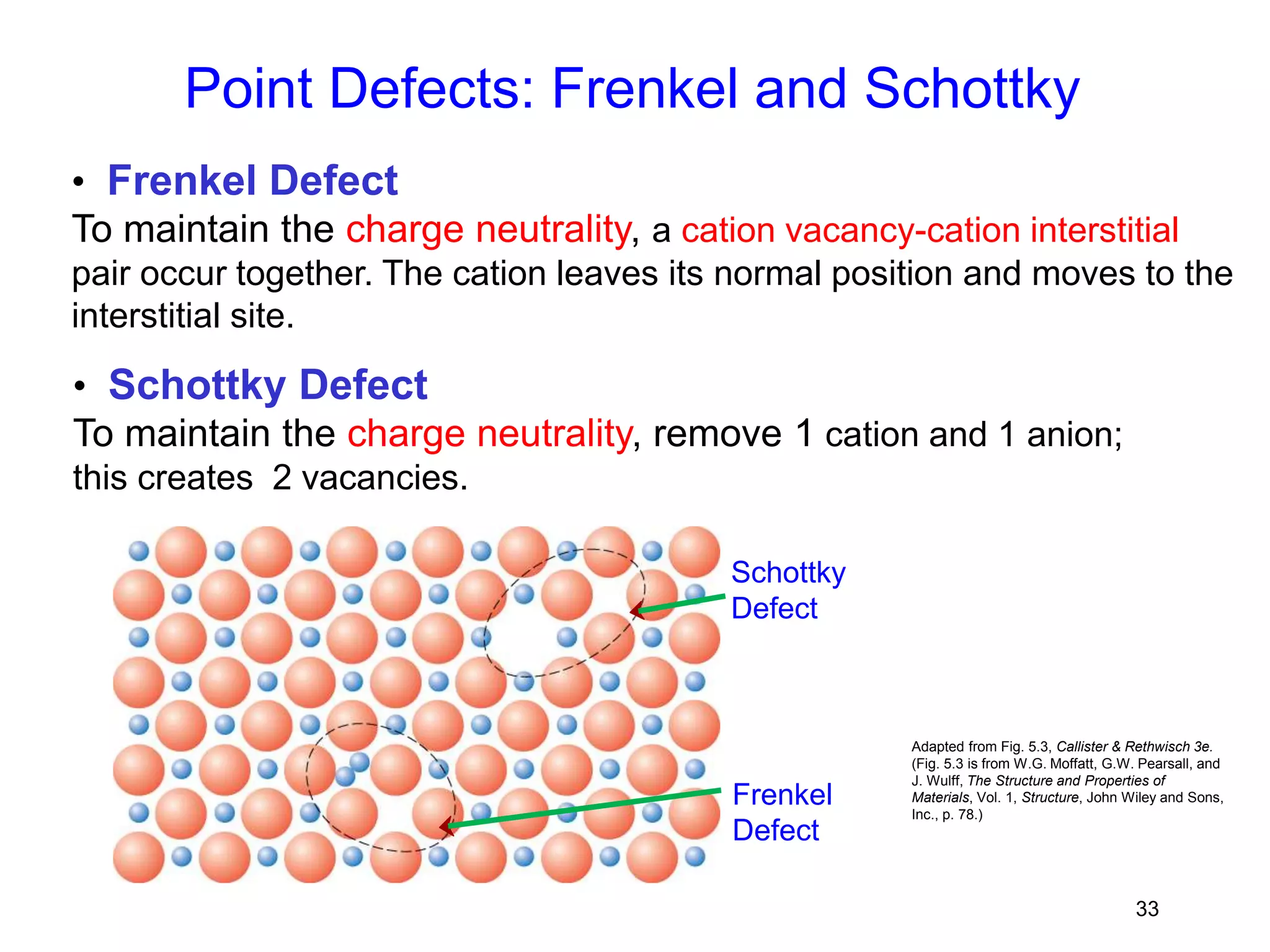
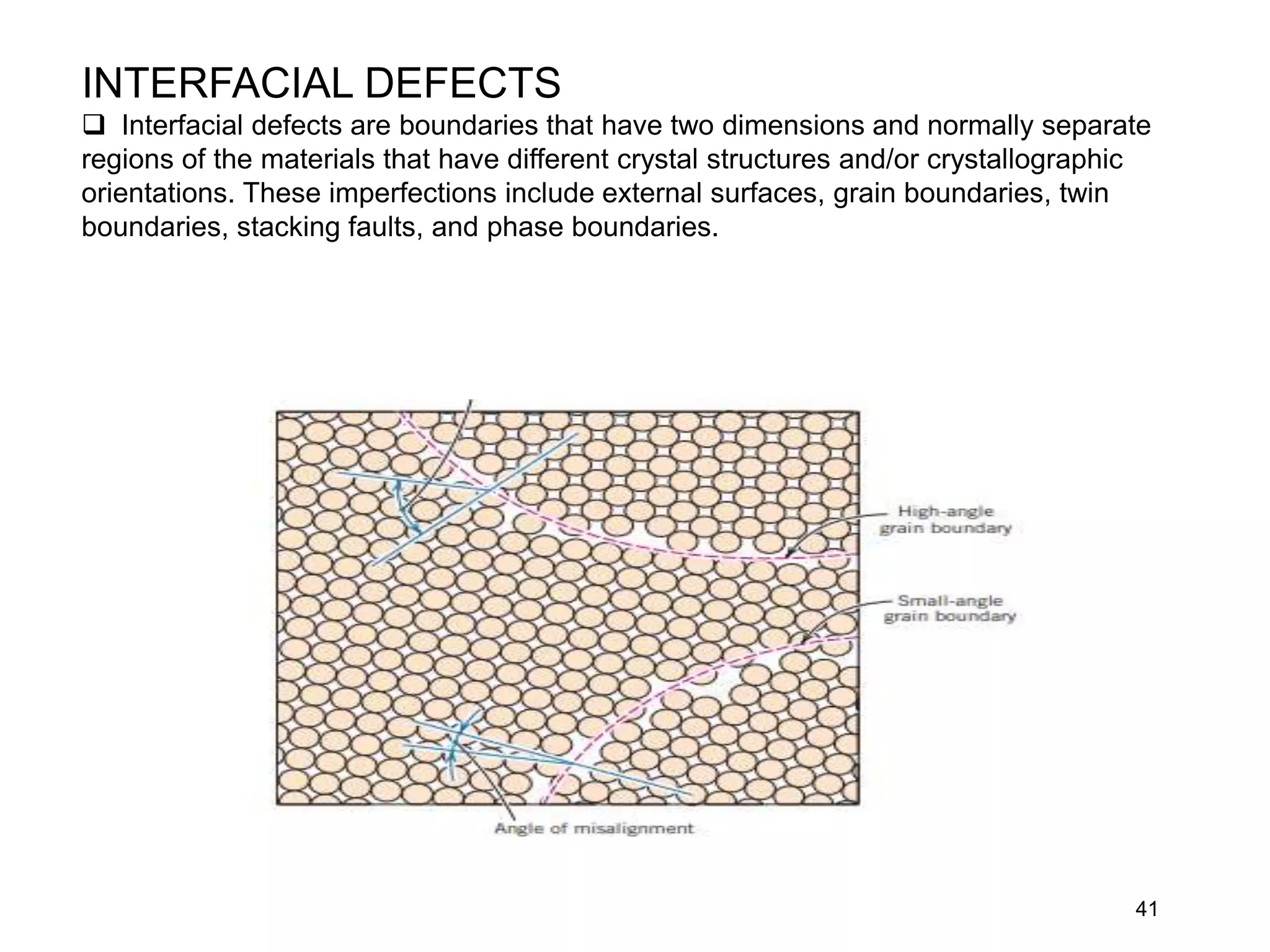
1. There are three main types of crystal imperfections - point defects, line defects, and interfacial defects. Point defects involve atoms being missing or in irregular positions in the lattice. Line defects are groups of atoms in irregular positions known as dislocations. Interfacial defects are boundaries separating regions with different crystal structures.
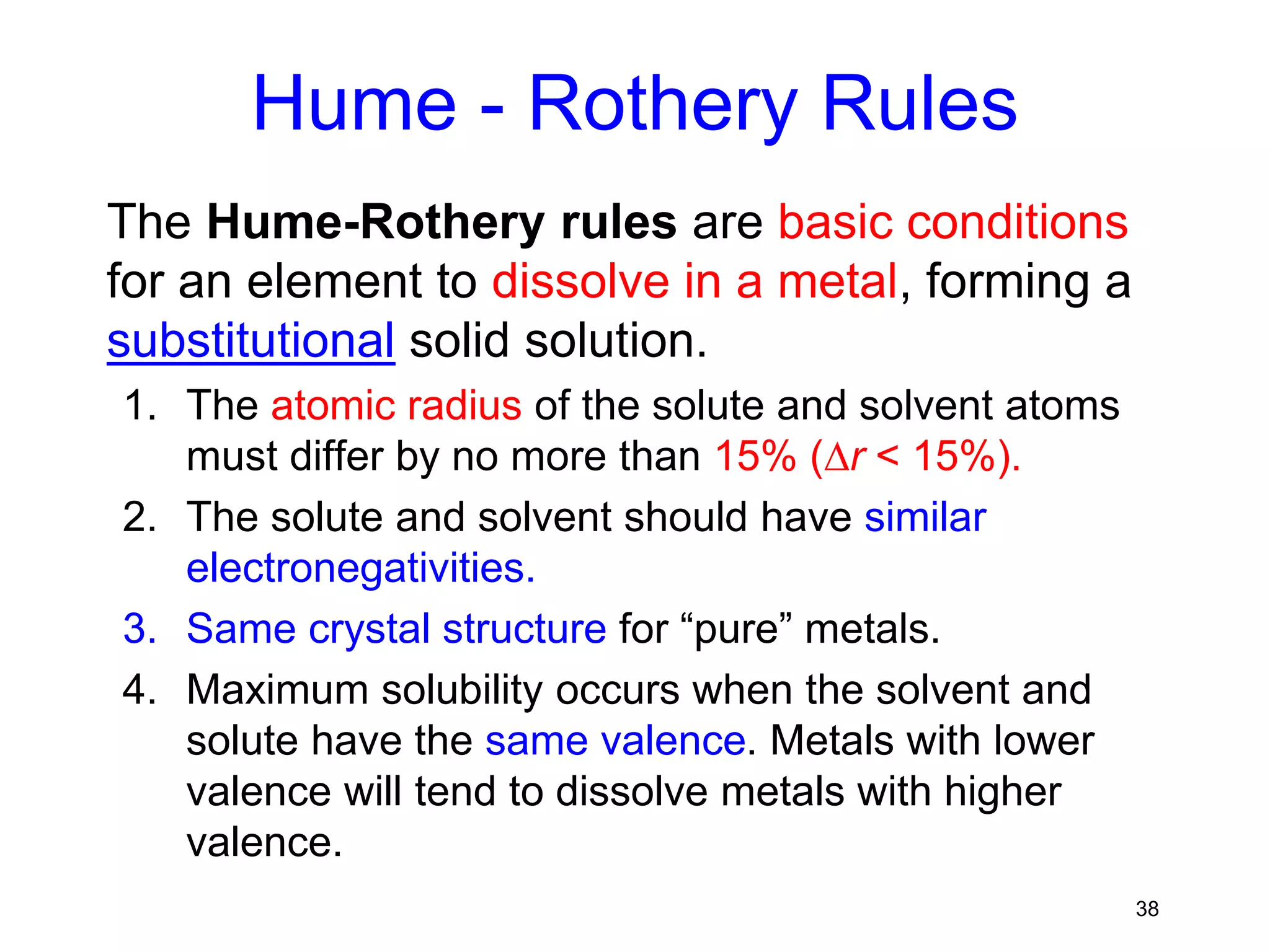
2. Solid solutions form when solute atoms are added to a solvent metal and the crystal structure is maintained. Factors like atomic radius, electronegativity, and crystal structure determine if a substitutional or interstitial solid solution forms.
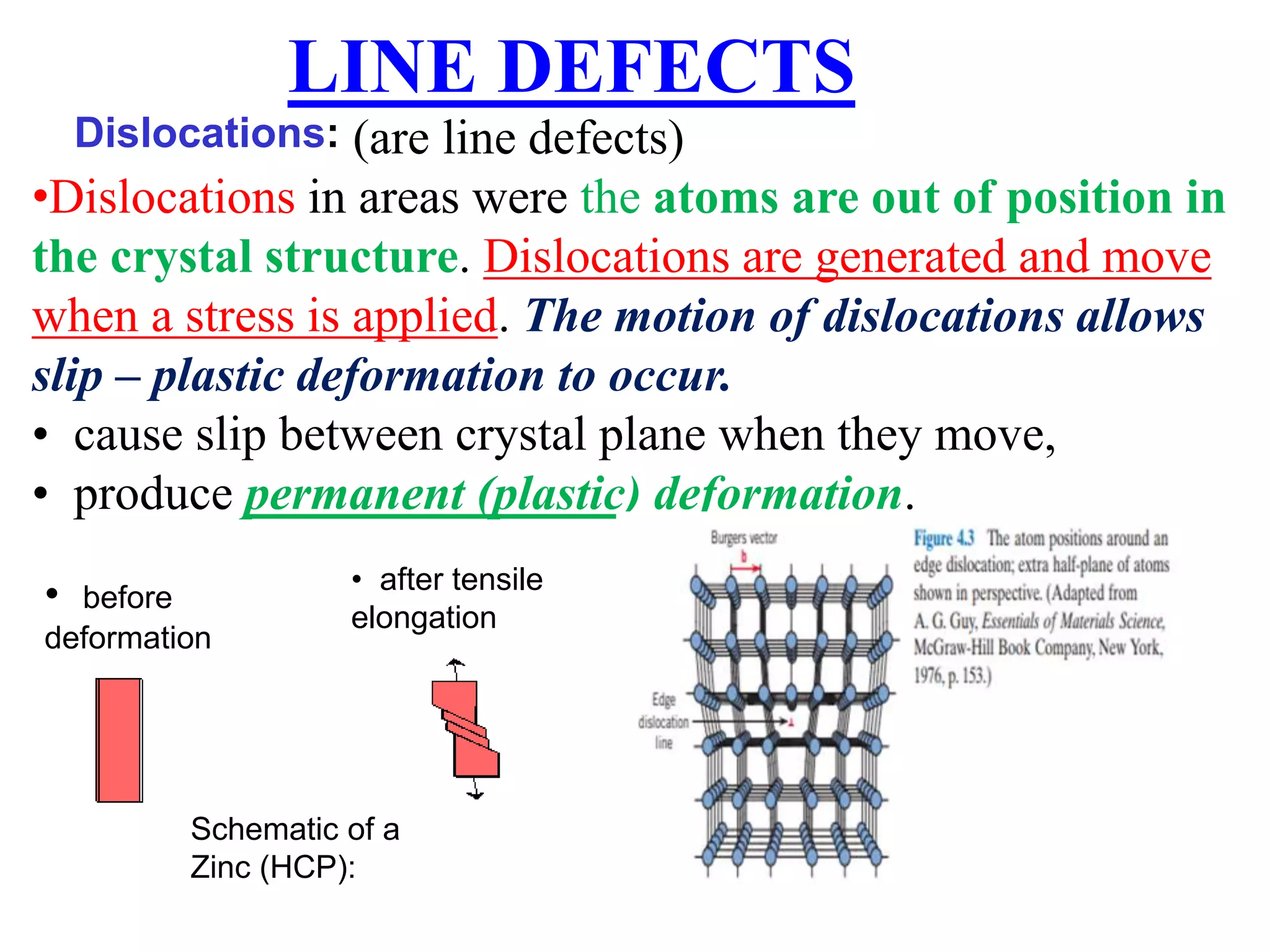
3. Dislocations are line defects that allow plastic deformation to occur when they move in response to stress. Their motion produces slip between crystal planes