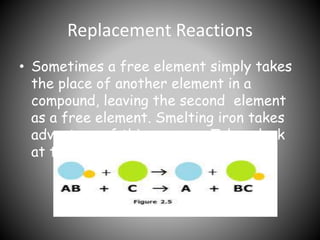
This document discusses different types of chemical reactions including synthesis reactions where substances join to form new compounds, decomposition reactions where compounds break down into simpler substances, replacement reactions where one element replaces another in a compound, and combustion reactions where oxygen combines with other compounds to form water and carbon dioxide. It also describes exothermic reactions which release heat and endothermic reactions which absorb heat. Neutralization reactions involve acids and bases canceling each other out.