Embed presentation
Download to read offline



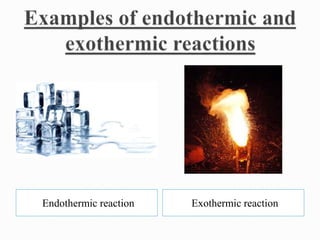
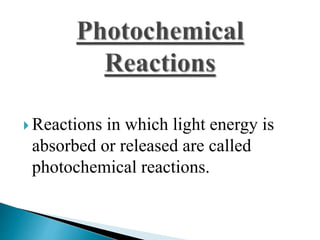
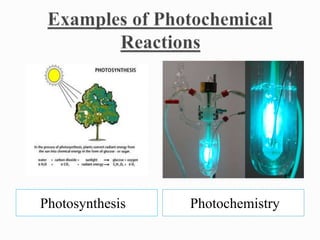

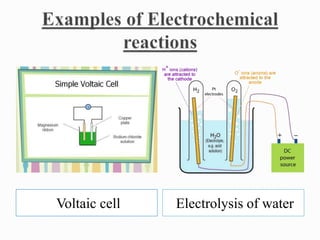

Physical changes involve temporary, reversible changes to a substance without producing new substances, such as melting or evaporation, while chemical changes are permanent and involve new substances forming, like burning or rusting. Reactions are also classified based on the type of energy absorbed or released, such as endothermic reactions absorbing heat, exothermic reactions releasing heat, photochemical reactions involving light, and electrochemical reactions relating to electrical energy.











