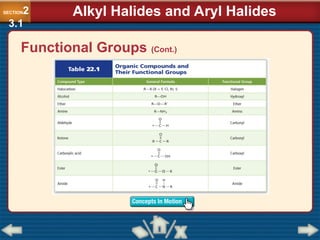
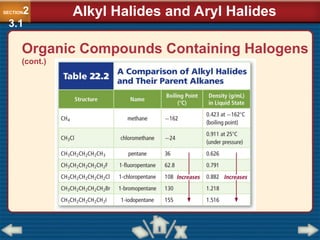
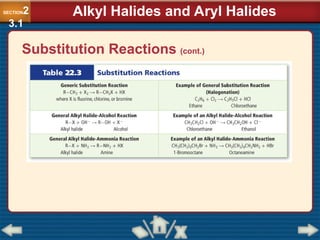
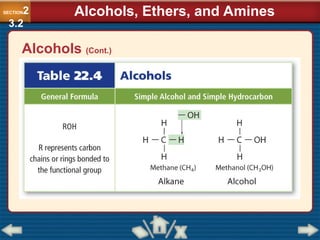
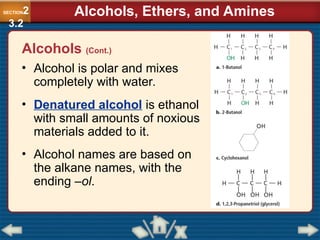
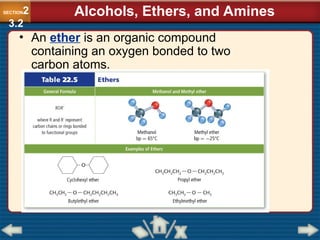
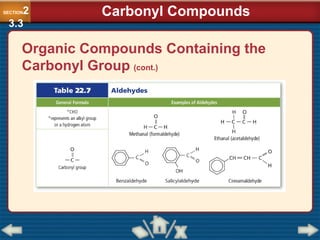
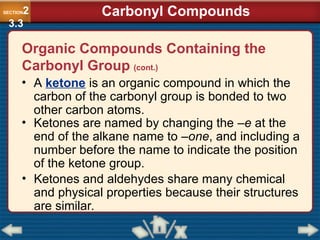
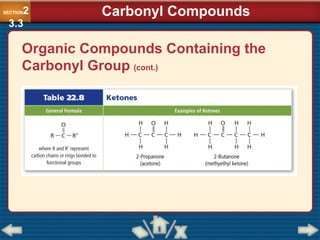
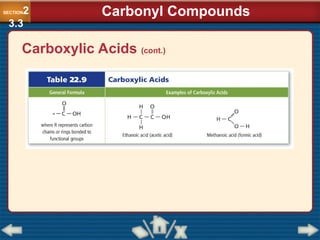
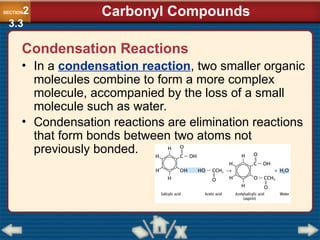
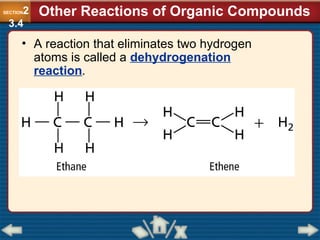
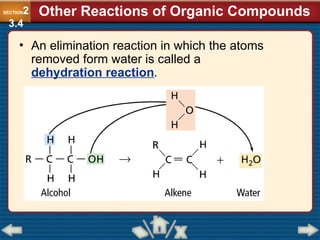
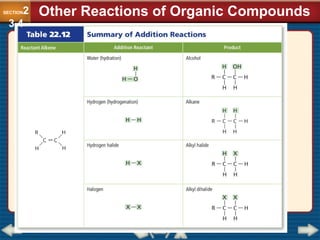
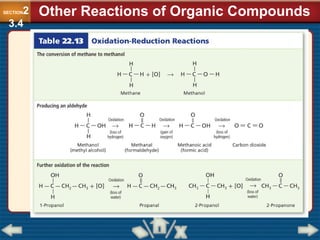
Chapter 23 discusses substituted hydrocarbons and their reactions, covering various functional groups such as alkyl halides, aryl halides, alcohols, ethers, amines, carbonyl compounds, and polymers. It explains the process of substitution reactions, the properties of these organic compounds, and their classification into categories like substitution, addition, and condensation reactions. The chapter emphasizes the importance of functional groups in determining the chemical and physical properties of organic compounds.