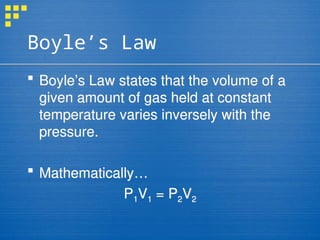
The kinetic molecular theory (KMT) describes the behavior of gases, highlighting that gas particles are in constant motion, collide without losing energy, and are separated by significant distances. It explains gas pressure as the result of these collisions with container walls and outlines key laws of gas behavior, including Boyle’s, Charles’s, and Gay-Lussac’s laws, which relate pressure, volume, and temperature. The document also discusses the differences between ideal and real gases, noting that real gases can exhibit attractions and occupy volume, particularly under high pressure and low temperature conditions.