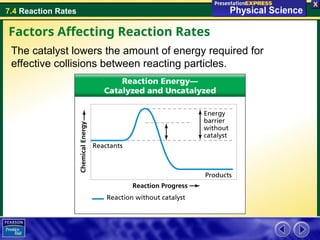
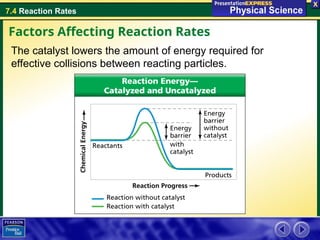
The document explains the concept of reaction rates, defined as the speed at which reactants convert into products over time. It discusses factors that influence these rates, including temperature, surface area, concentration, stirring, and catalysts. Additionally, it provides examples of how these factors can accelerate or decelerate reactions, such as the use of catalysts in chemical processes.