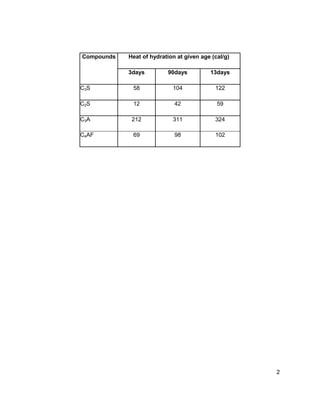
Cement hydration is the chemical reaction between cement and water. When cement compounds are mixed with water, they dissolve and form a supersaturated solution. Hydrated compounds then precipitate out of the solution. The main hydrated compounds have low solubility and are responsible for the setting and hardening of cement. The quality, quantity, and rate of formation of these hydrated compounds affect the properties of the hardened cement.













![2
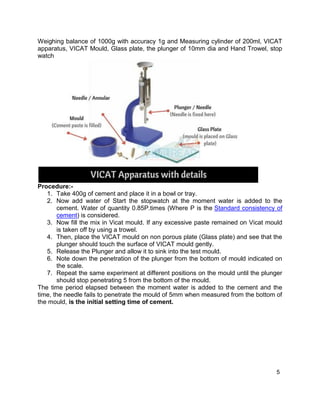
About Lechatelier mould:
It consists of a small split cylinder forming a mould having dimensions of internal dia
30mm and height 30mm. On either side of the split cylinder, two parallel indicating arms
with pointed ends of length 165mm is attached.
Remember, Lechatlier mould is a split cylinder (opened Cylinder)
Indicator arms determine the expansion of cement.
Procedure:
1. Before Performing the test, calculate the standard consistency of cement to
find out the water required to obtain the normal consistency(P).
2. Now add 0.78 times of water to the cement to give a paste of standard
consistency (0.78P).
3. Lightly apply oil to the Lechatelier mould and place it on a glass plate.
4. Now pour the cement paste into mould and close the mould using lightly oiled
glass plate and to avoid misplacement place a weight on it.
5. Then, submerge the whole assembly for 24Hrs in water bath at a temperature of
270
C
6. Remove the entire apparatus from water and then calculate the distance
separating two indicator points using measuring scale and note it as L1.
7. Again submerge the whole assembly in a water bath at a temperature of boiling
point for 3hours.
8. After completion of 3 hours remove the assembly from the bath and measure the
distance between two indicator points and note it as L2.
How do indicator arms determine expansion of cement?
Well, as mentioned above the mould is split cylinder which means that, if cement starts
expanding the length is increased by calculating the difference between the two lengths
the expansion of cement is found.
Expansion of Cement = L1-L2
A good cement (OPC, PPC, Rapid hardeneing etc) should have not more than below
expansion limits.
Types of Cement
Expansion
Limits
Ordinary Portland cement
[OPC]
33 Grade, 43 Grade, 53 grade
10mm
Portland Pozzolona Cement
[PPC]
10mm
Rapid Hardening cement 10mm
Low heat cement 10mm
Super sulphated cement 5mm](https://image.slidesharecdn.com/uniti-220528030050-3bb7c24d/85/Concrete-Technology-16-320.jpg)




![8
Test Result
The test result should be 5-7 mm measurement in Vicat apparatus.
Compressive Strength of Cement
Compressive strength is the capacity of material or structure to resist or withstand under
compression. The compressive strength of a material is determined by the ability of the
material to resist failure in the form of cracks and fissure.
In this test, the impact force applied on both faces of mortar specimen made with
cement and the maximum compression that cement specimen bears without failure
recorded.
In technical terms compressive strength of cement means,
The ability of cement specimen to resist the compressive stress when tested
under Compressive Testing Machine [CTM] at 28 days.
Apparatus:
The apparatus required for testing Compressive Strength of cement is
1. Compressive Test machine conforms to IS: 14858(2000)
2. Steel cubes of 7.06cm moulds (50cm2
face area) conforming to IS: 10080-1982,](https://image.slidesharecdn.com/uniti-220528030050-3bb7c24d/85/Concrete-Technology-22-320.jpg)