Embed presentation
Downloaded 24 times
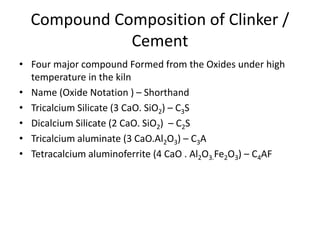
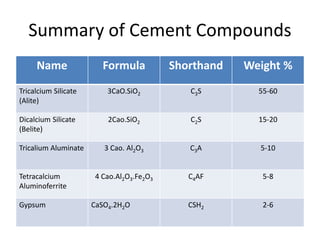

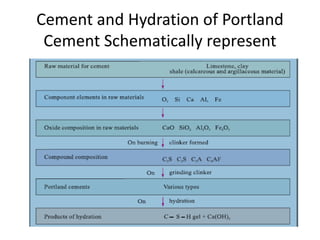


The document discusses the major compounds found in cement clinker and their abbreviations. The four major compounds are tricalcium silicate (C3S), dicalcium silicate (C2S), tricalcium aluminate (C3A), and tetracalcium aluminoferrite (C4AF). C3S contributes to early strength while C2S defines later strength. C3A reacts immediately with water but causes flash setting without gypsum. Hydration of the cement forms calcium silicate hydrate (C-S-H) and calcium hydroxide, which are the primary products that give cement its strength.




