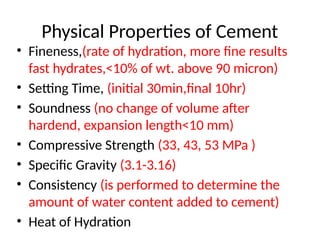
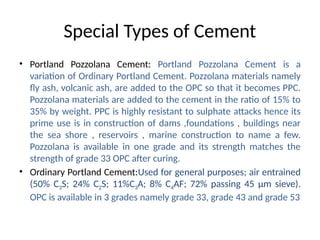
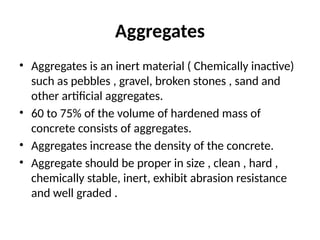
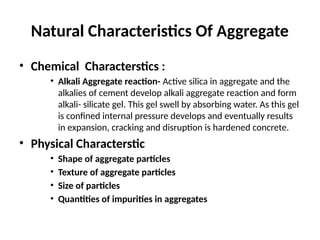
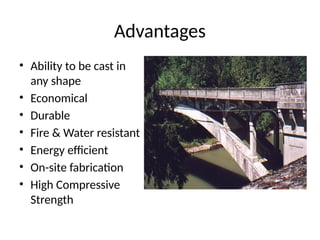
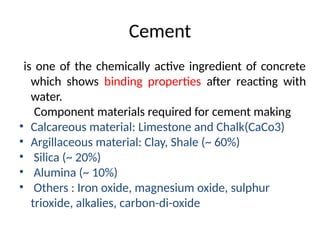
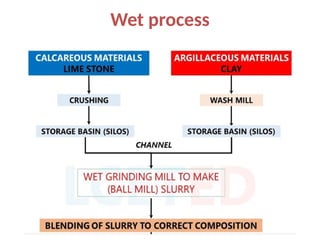
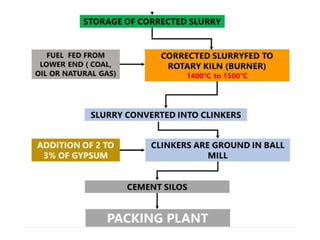
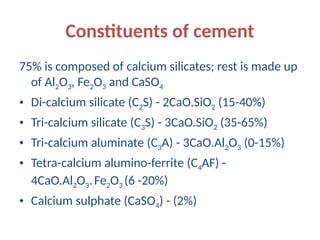
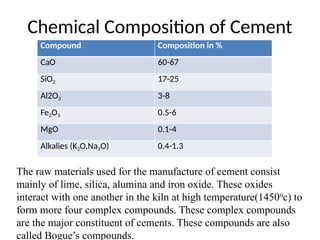
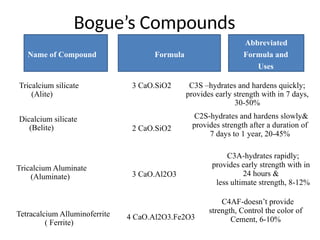
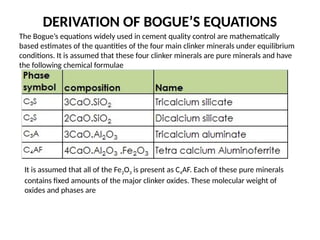
The document outlines the properties, advantages, disadvantages, and manufacturing process of concrete, highlighting its constituents such as cement, aggregates, and water. It provides detailed information on different types of cements and their specific uses, as well as the process of hydration that gives concrete its strength. Additionally, the document discusses the importance of aggregates in concrete composition and their classifications based on various criteria.












![Rearranging this equation we get;-
C3A = (Al2O3 – 0.6386 x Fe2O3) / 0.3774
C3A = 2.650 x Al2O3 – 1.692 x Fe2O3 Which is the Bogue equation for C3A
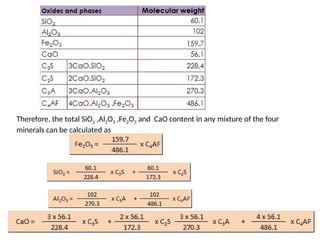
Substituting the values of C2S ,C3A and C4AF in CaO equation
CaO = (0.7369 x C3S) + (0.6512 x C2S) + (0.6226 x (2.650 x Al2O3 – 1.692 x Fe2O3)) + (0.4616
x 3.044 x Fe2O3 )
CaO = (0.7369 x C3S) + (0.6512 x C2S) + (1.650 x Al2O3 ) – (1.053 x Fe2O3) + (1.405 x Fe2O3)
CaO = (0.7369 x C3S) + (0.6512 x C2S) + (1.650 x Al2O3) + (0.3520 x Fe2O3)
C3S = [CaO – (0.6512 x C2S) – (1.650 x Al2O3) – (0.352 x Fe2O3 )] / 0.7369
C3S = (1.357 x CaO) – (0.8837 x C2S) – (2.239 x Al2O3) – (0.4777 x Fe2O3)
Putting the value of C2S = (2.867 x SiO2) – (0.754 x C3S)
C3S = (1.357 x CaO) – {0.8837 x (2.867 x SiO2) – (0.754 x C3S)} – (2.239 x Al2O3) – (0.4777 x
Fe2O3)
C3S – (0.8837 x 0.754 x C3S) = (1.357 x CaO) – {0.8837 x (2.867 x SiO2)} – (2.239 x Al2O3) –
(0.4777 x Fe2O3)
0.3337C3S = 1.357 x CaO – 2.53 x SiO2 – 2.239 x Al2O3 – 0.4777 x Fe2O3
C3S = [1.357 x CaO – 2.53 x SiO2 – 2.239 x Al2O3 – 0.4777 x Fe2O3]/0.3337
C3S = 4.07 x CaO – 7.6 x SiO2 – 6.70 x Al2O3 – 1.42 x Fe2O3 Which is the Bogue equation
for C3S](https://image.slidesharecdn.com/concretetechnologychapter1-250120073116-41198d5b/85/Concrete-Technology-and-Masonry-StructureChapter-1-pptx-26-320.jpg)