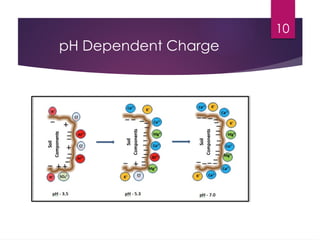
Cation Exchange Capacity (CEC) is the ability of soil to hold positively charged ions (cations) essential for plant nutrition, influenced by factors such as clay content, soil pH, and organic matter. Higher CEC indicates more clay and organic material, promoting soil fertility by retaining essential nutrients. Additionally, Anion Exchange Capacity (AEC) represents the capacity to hold negatively charged ions (anions) and is also dependent on soil pH.