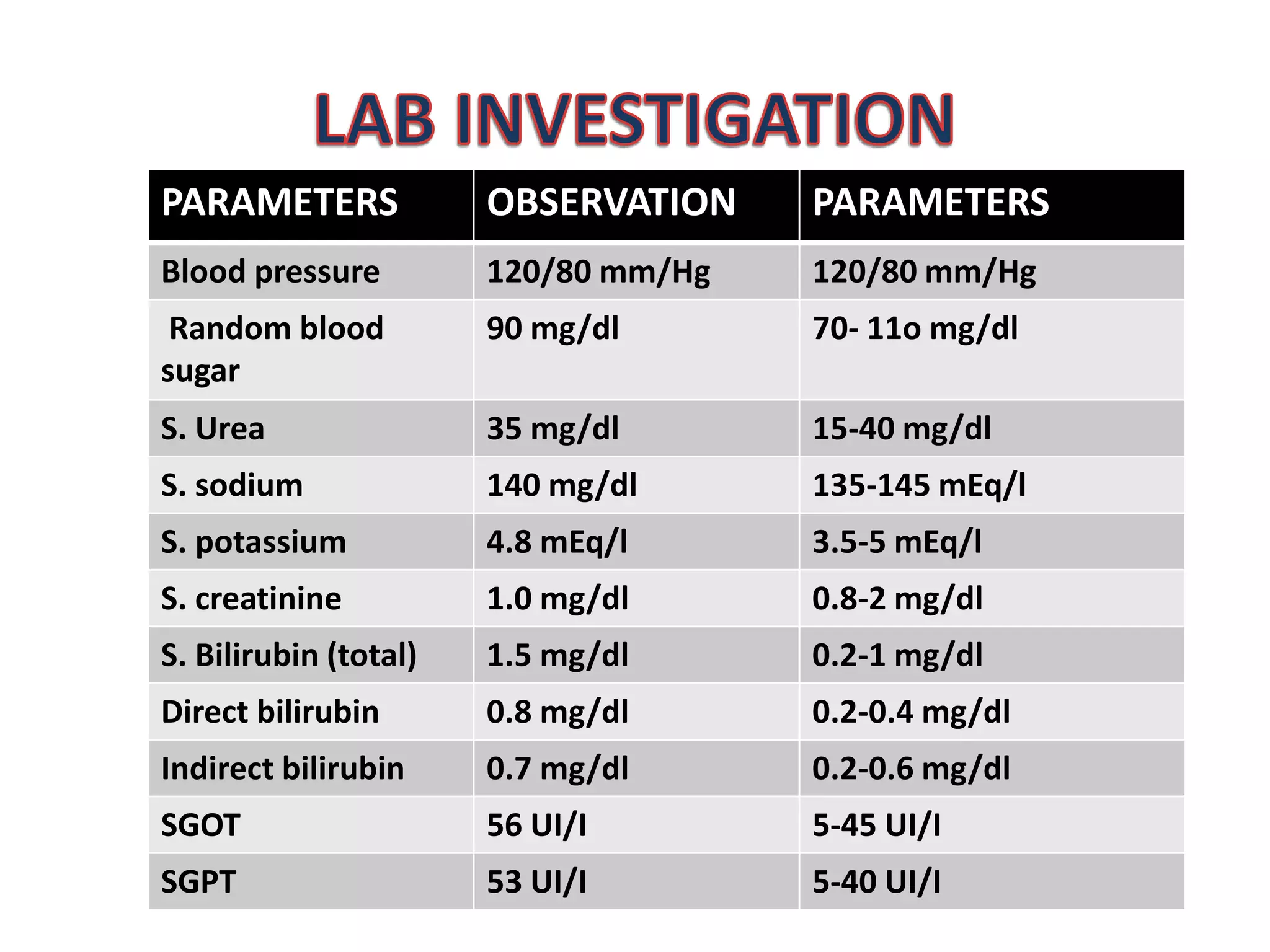
The patient, a 25-year-old male smoker, presented with a chief complaint of passing concentrated urine and yellow colored eyes and urine discharge for 15 days. Laboratory tests found elevated bilirubin levels. The patient was assessed with obstructive jaundice and prescribed injections of cefotaxime, ranitidine, prednisolone tablets, and Liv 52 tablets to treat the condition and its symptoms while addressing any potential drug interactions between the medications and the patient's liver condition.