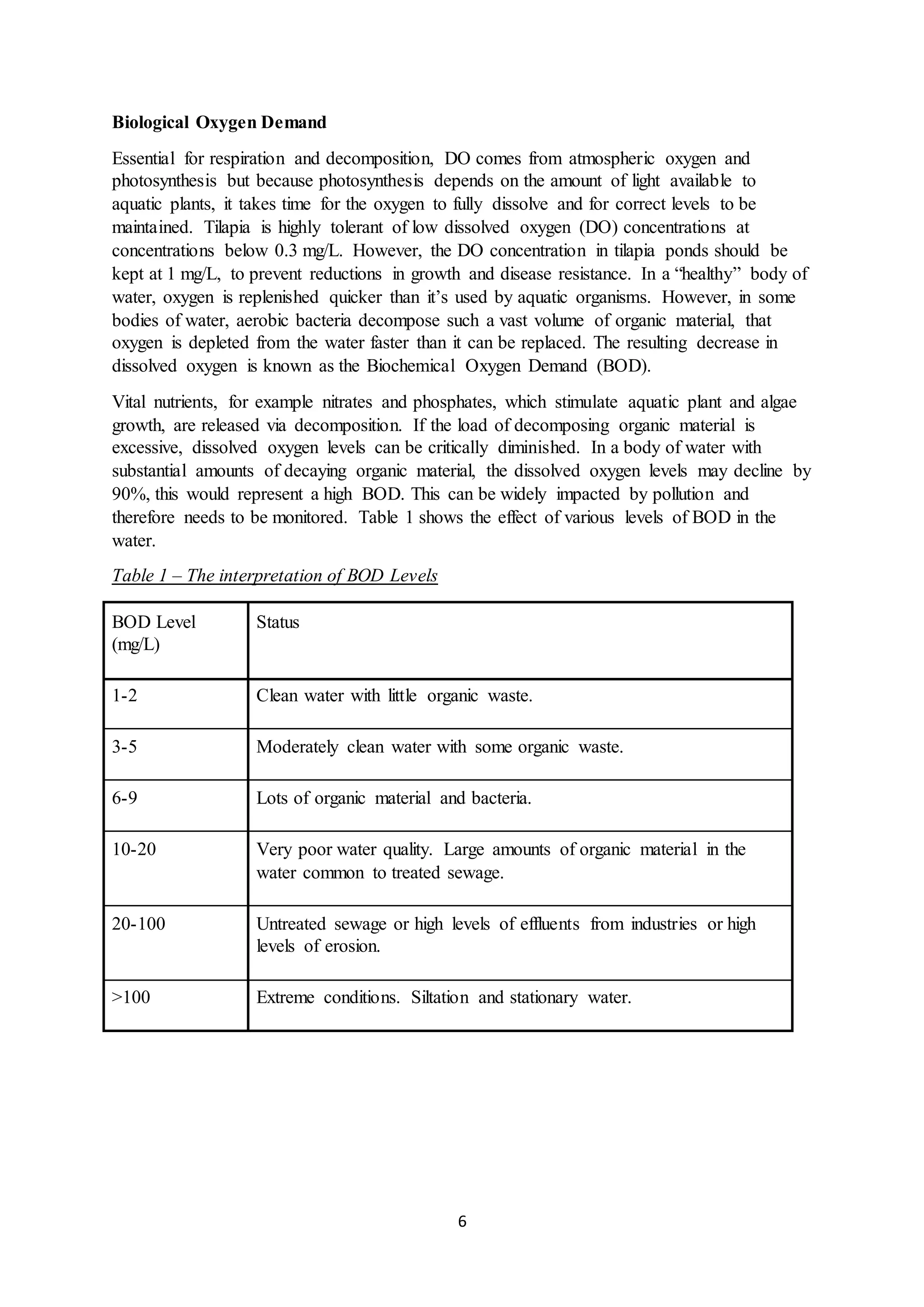
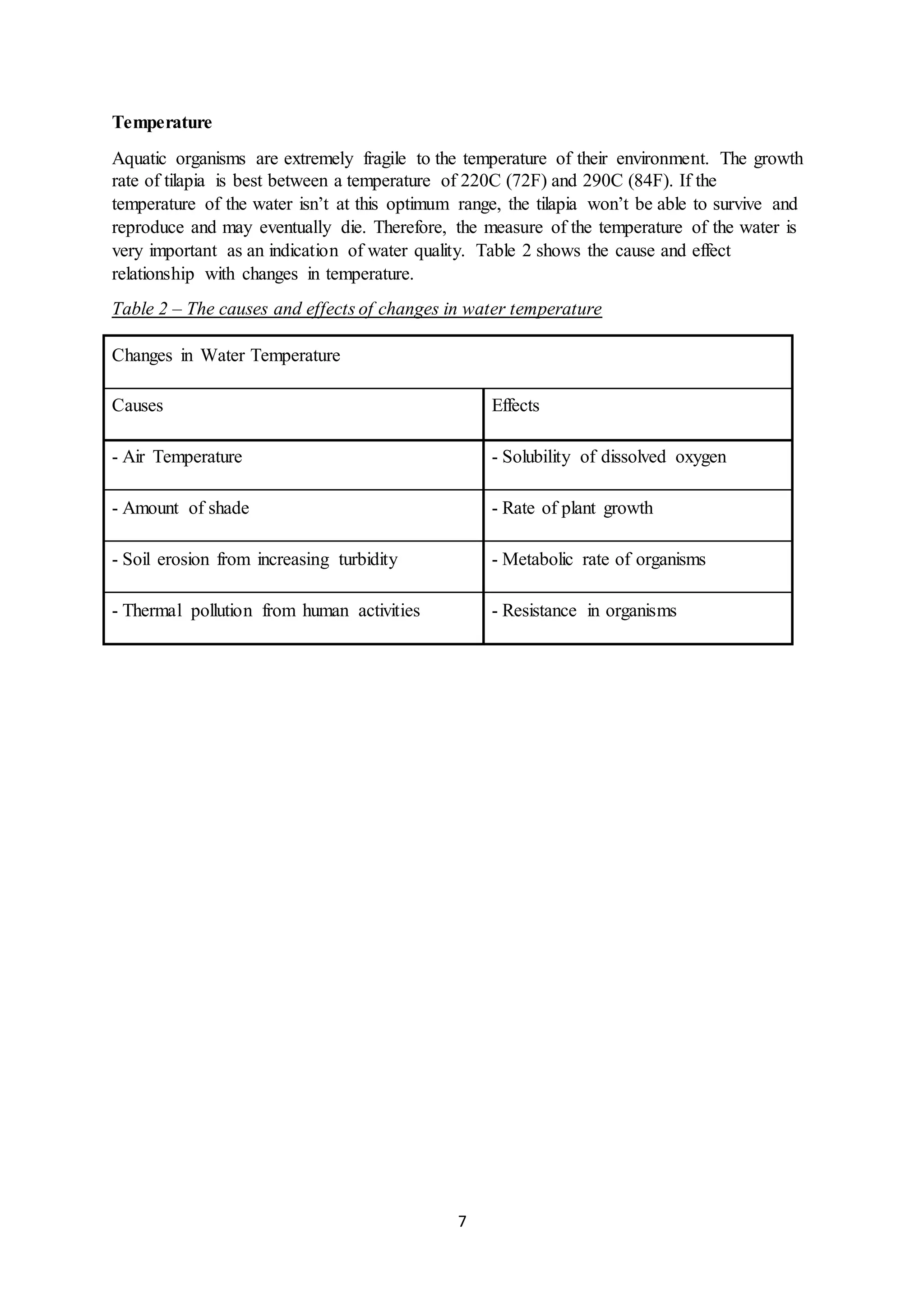
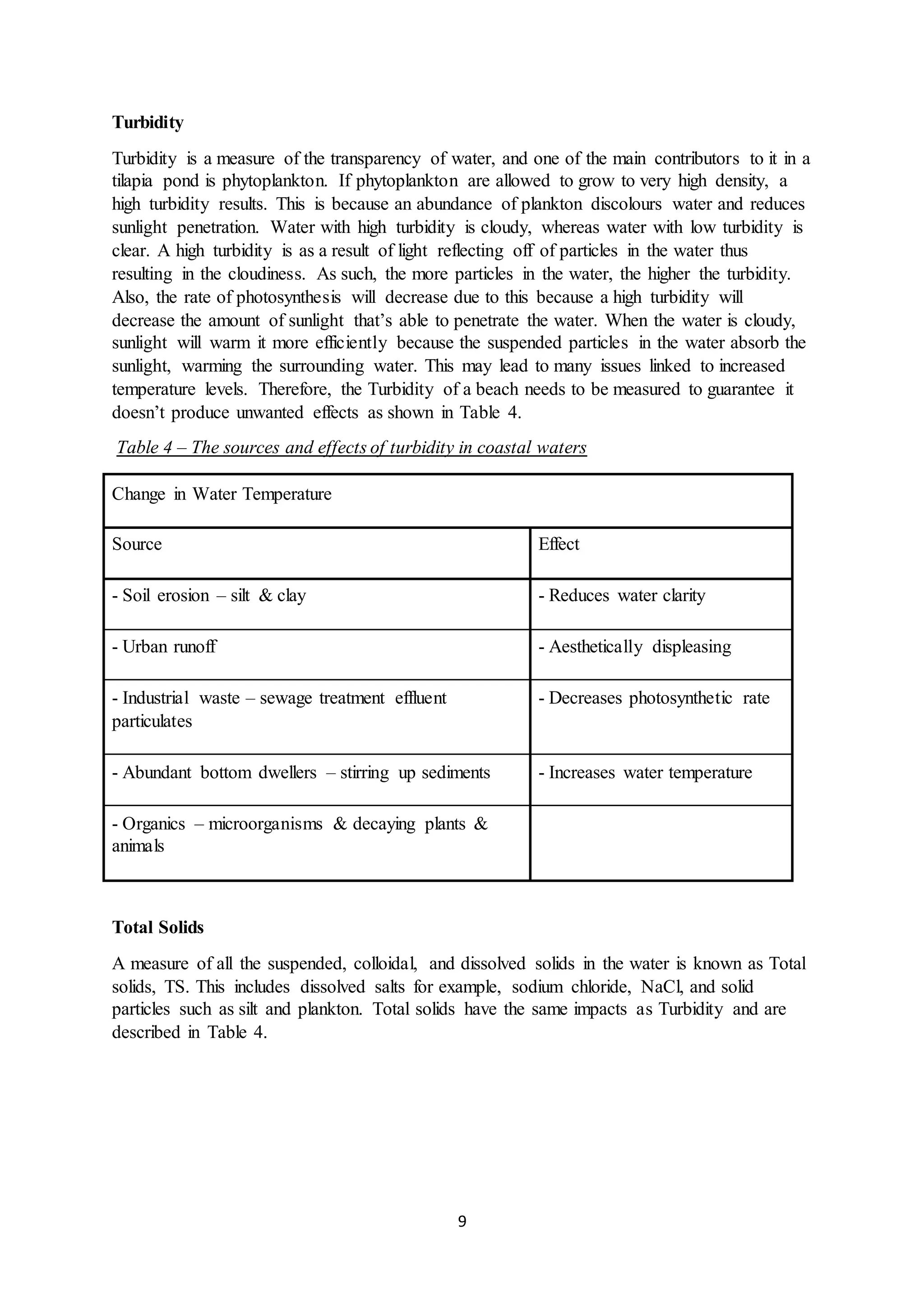
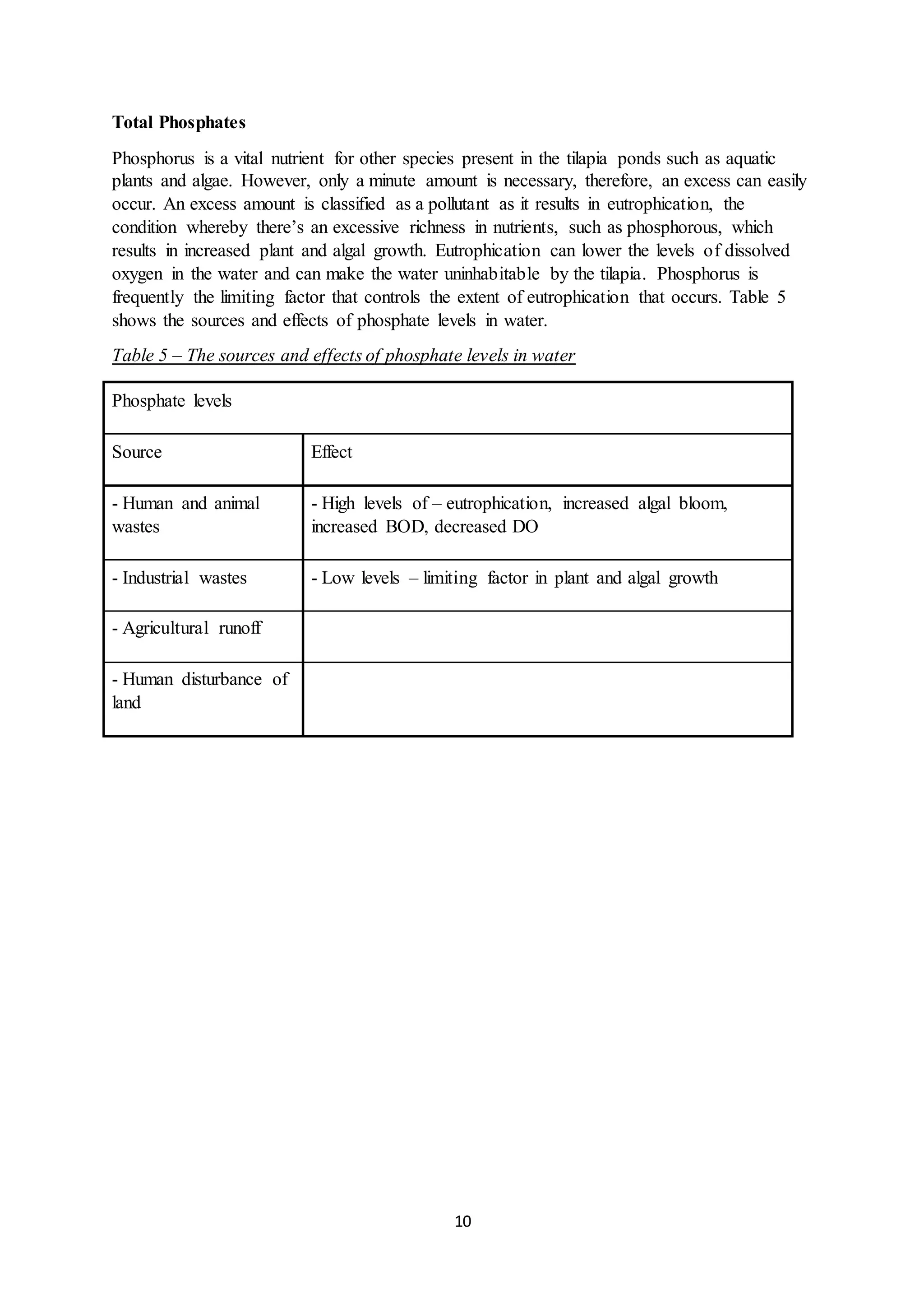
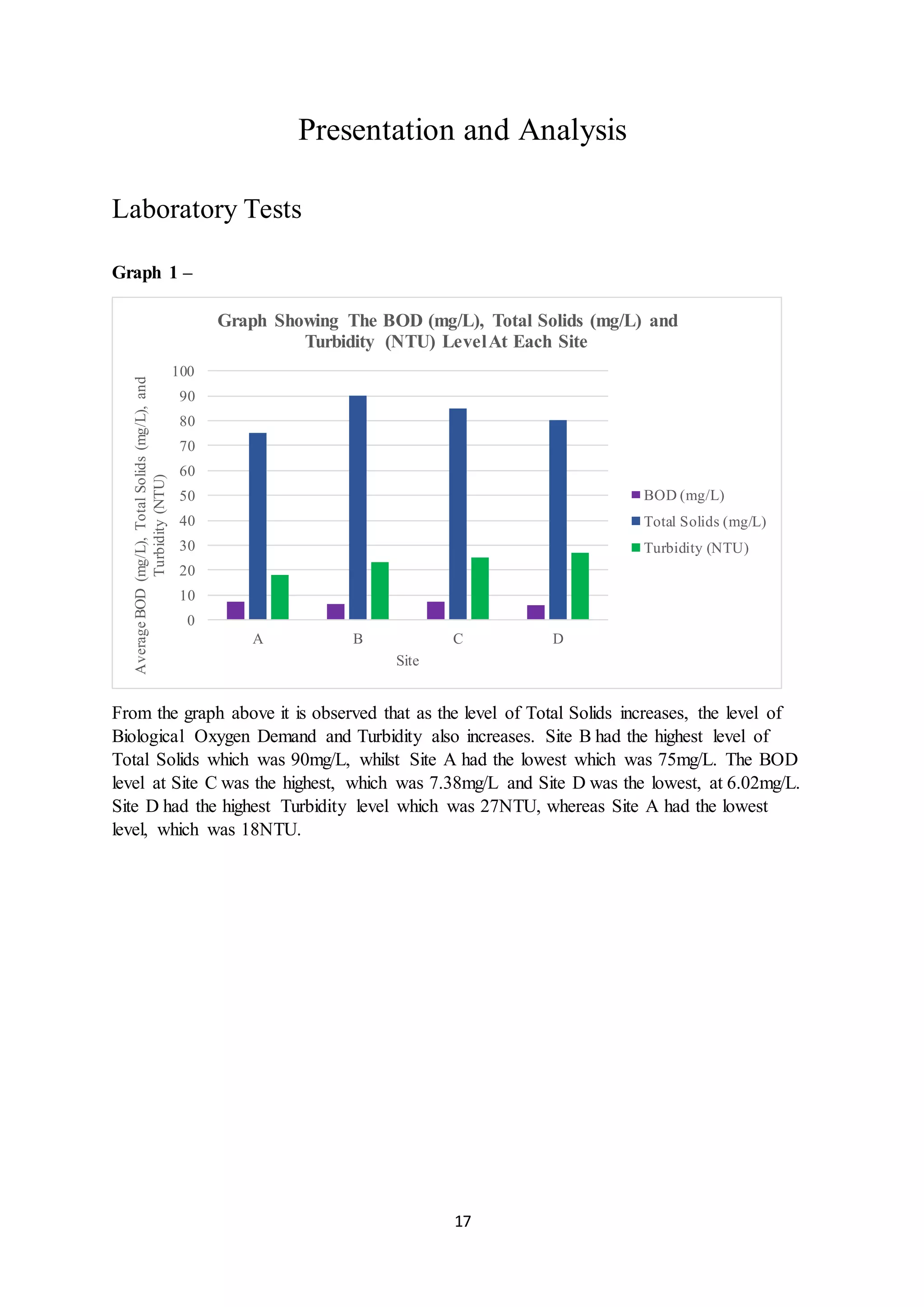
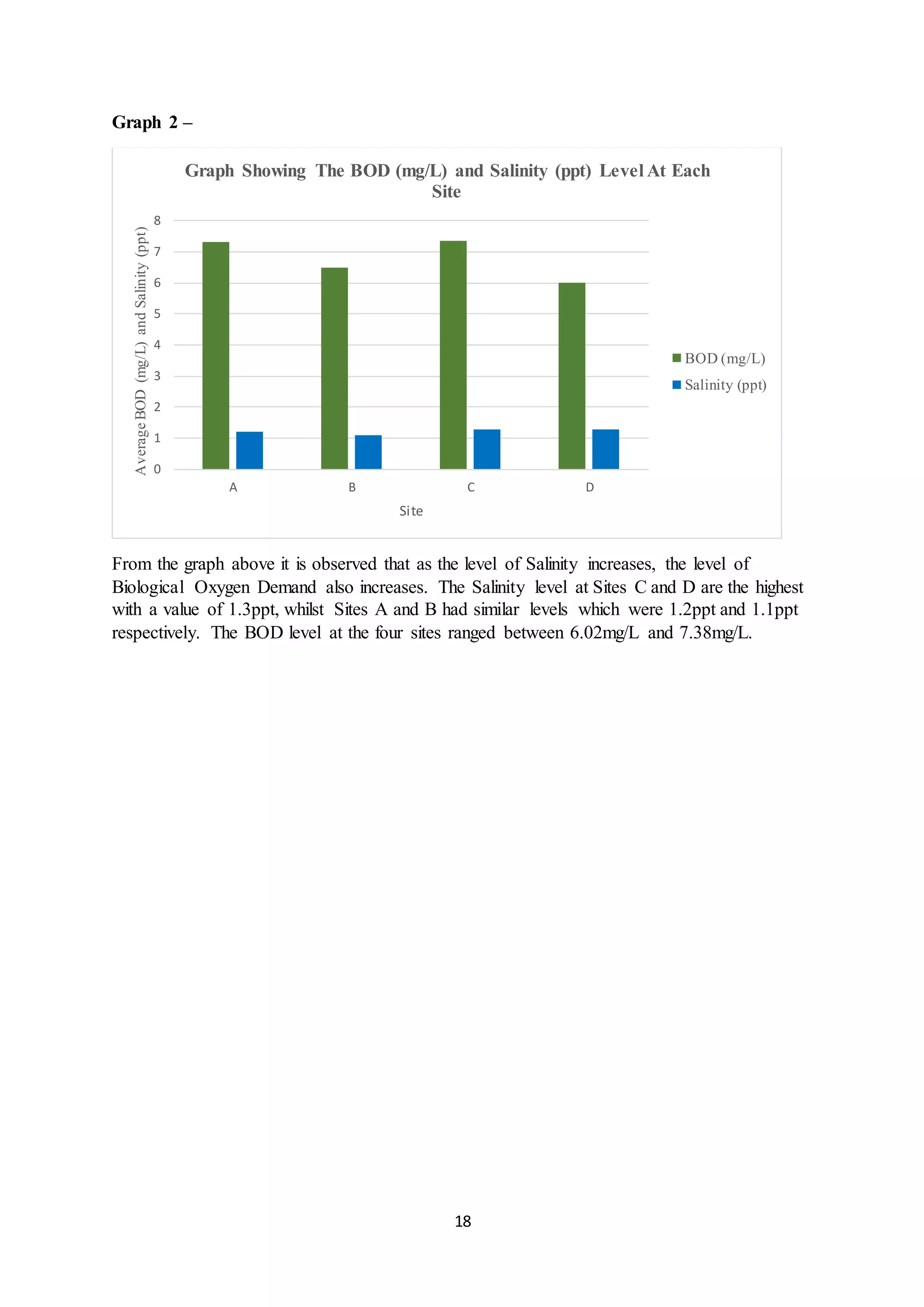
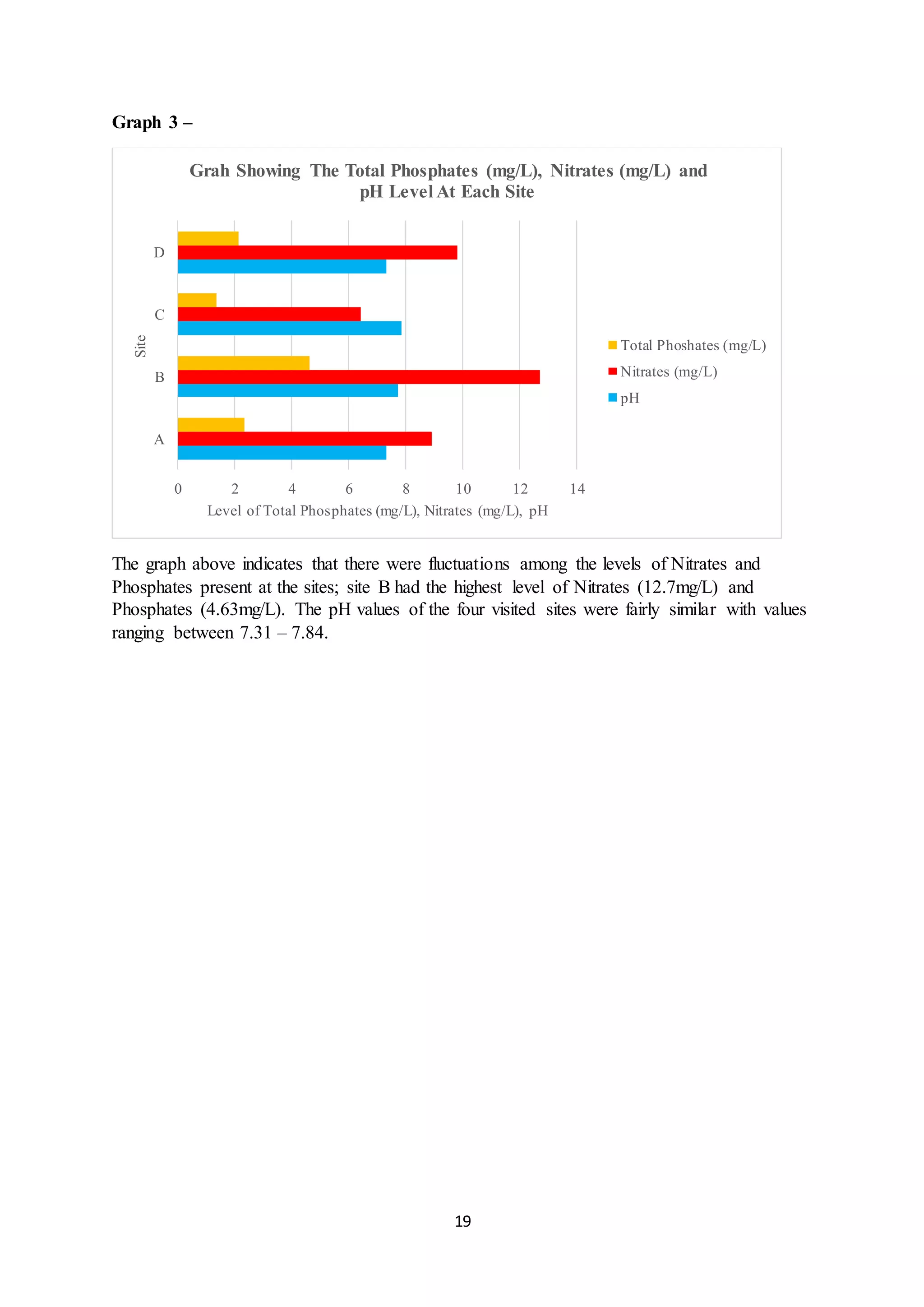
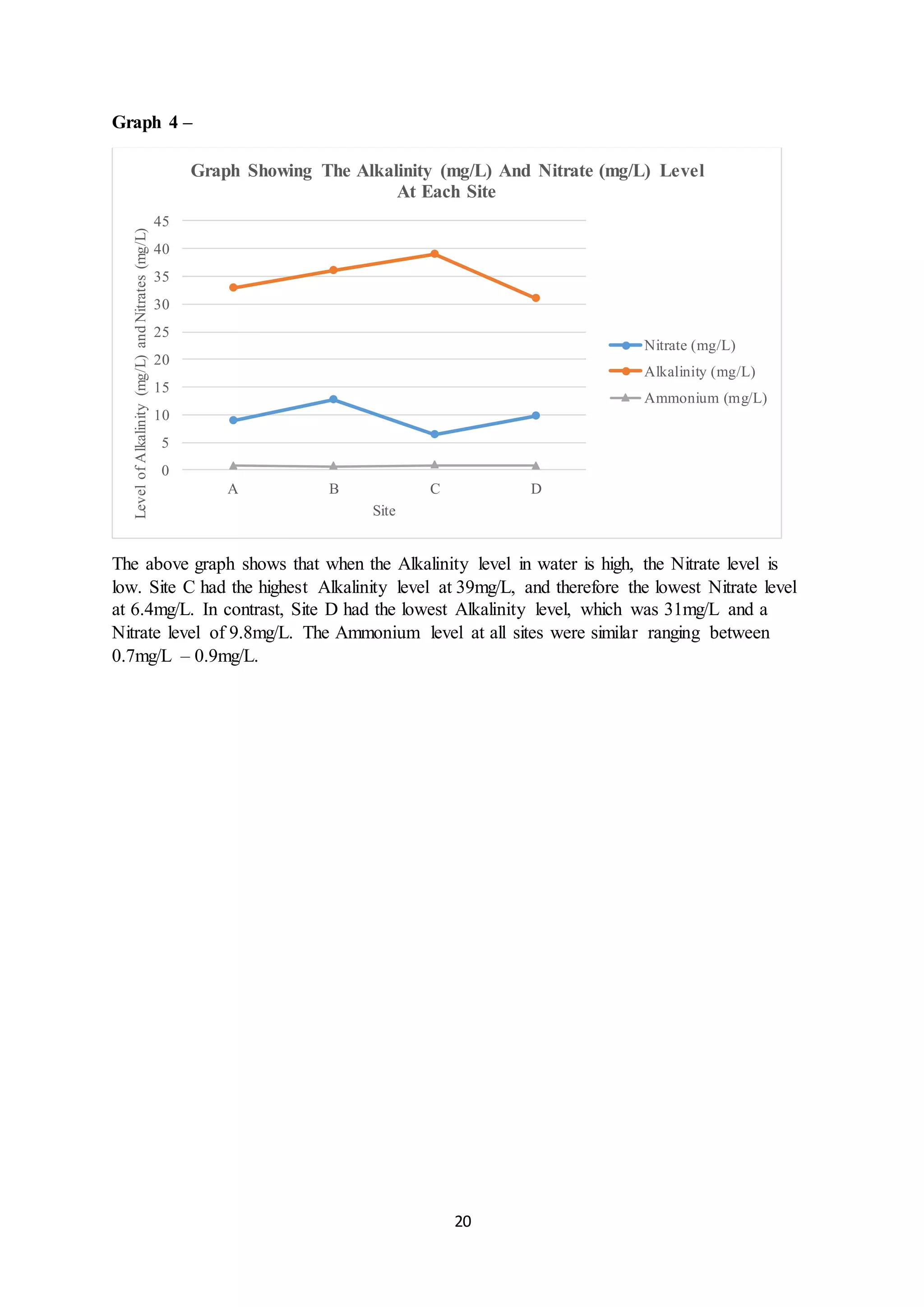
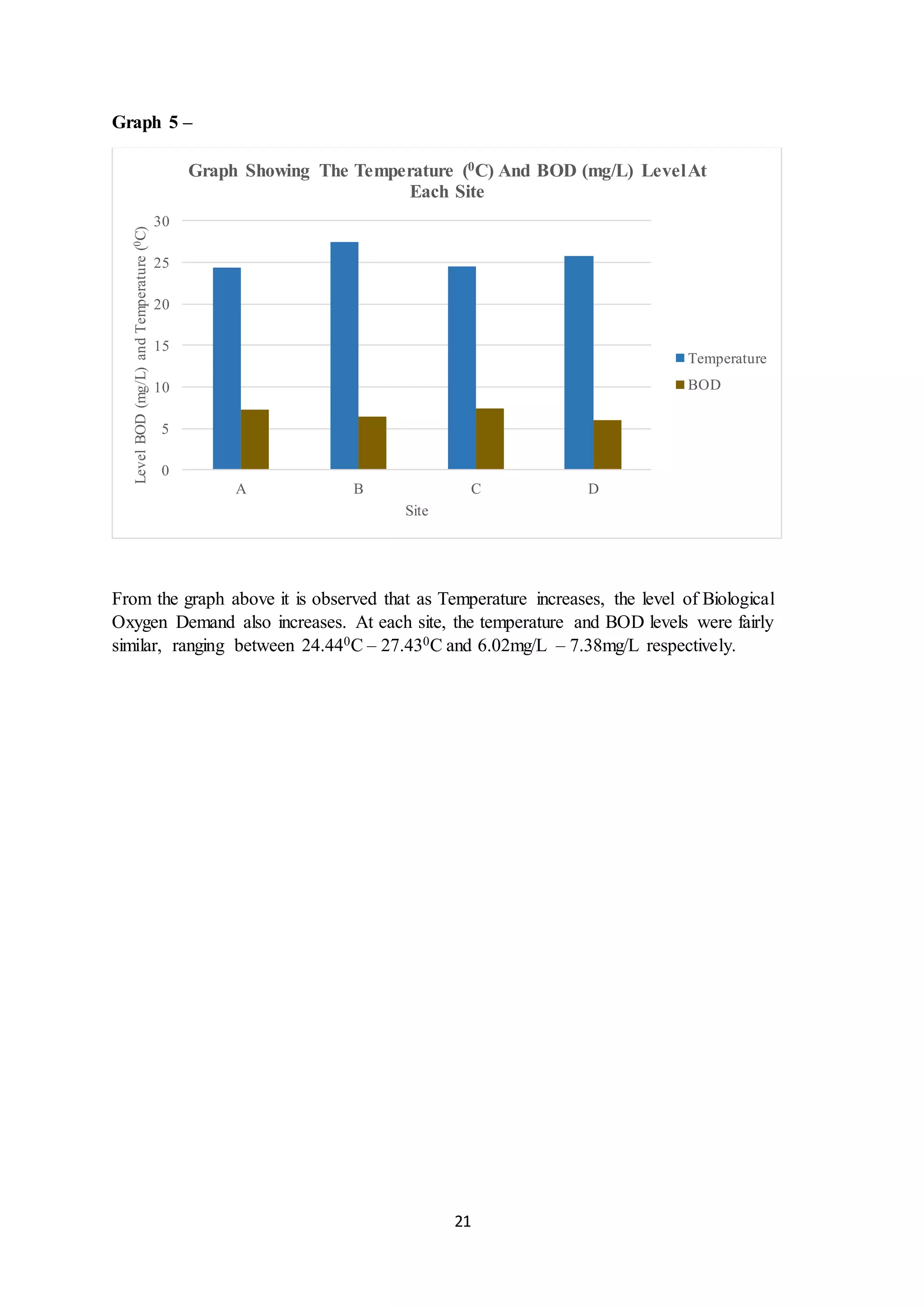
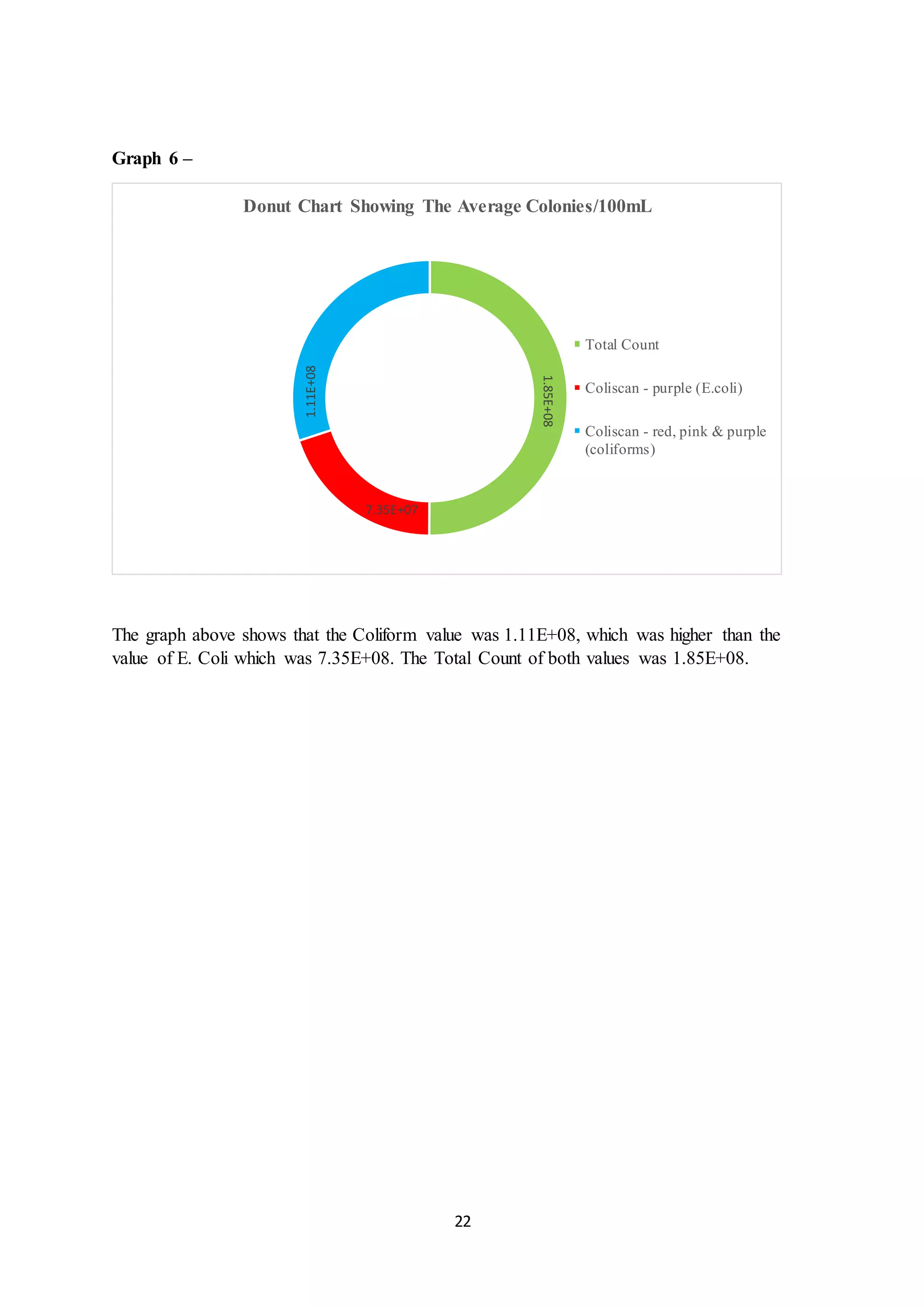
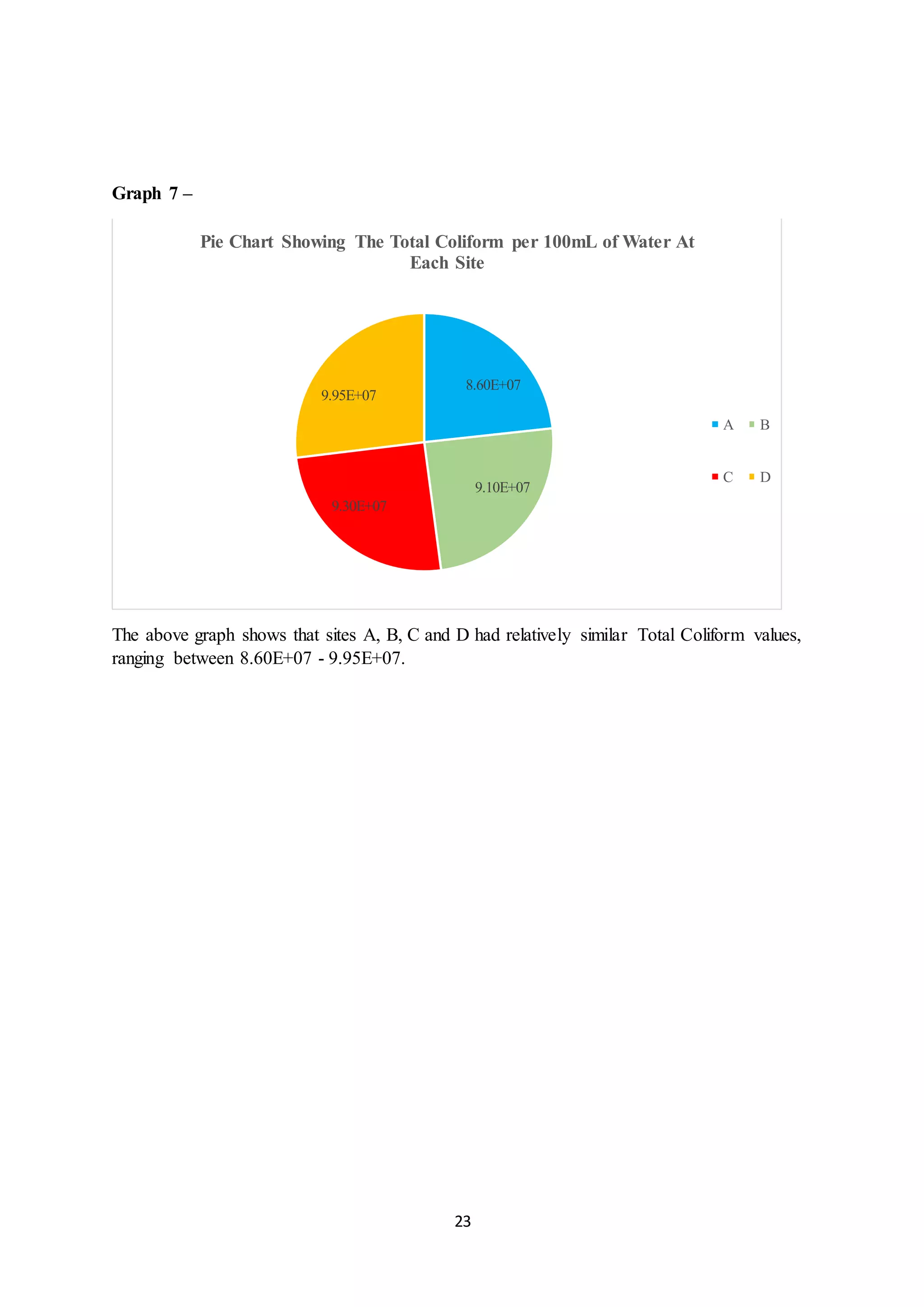
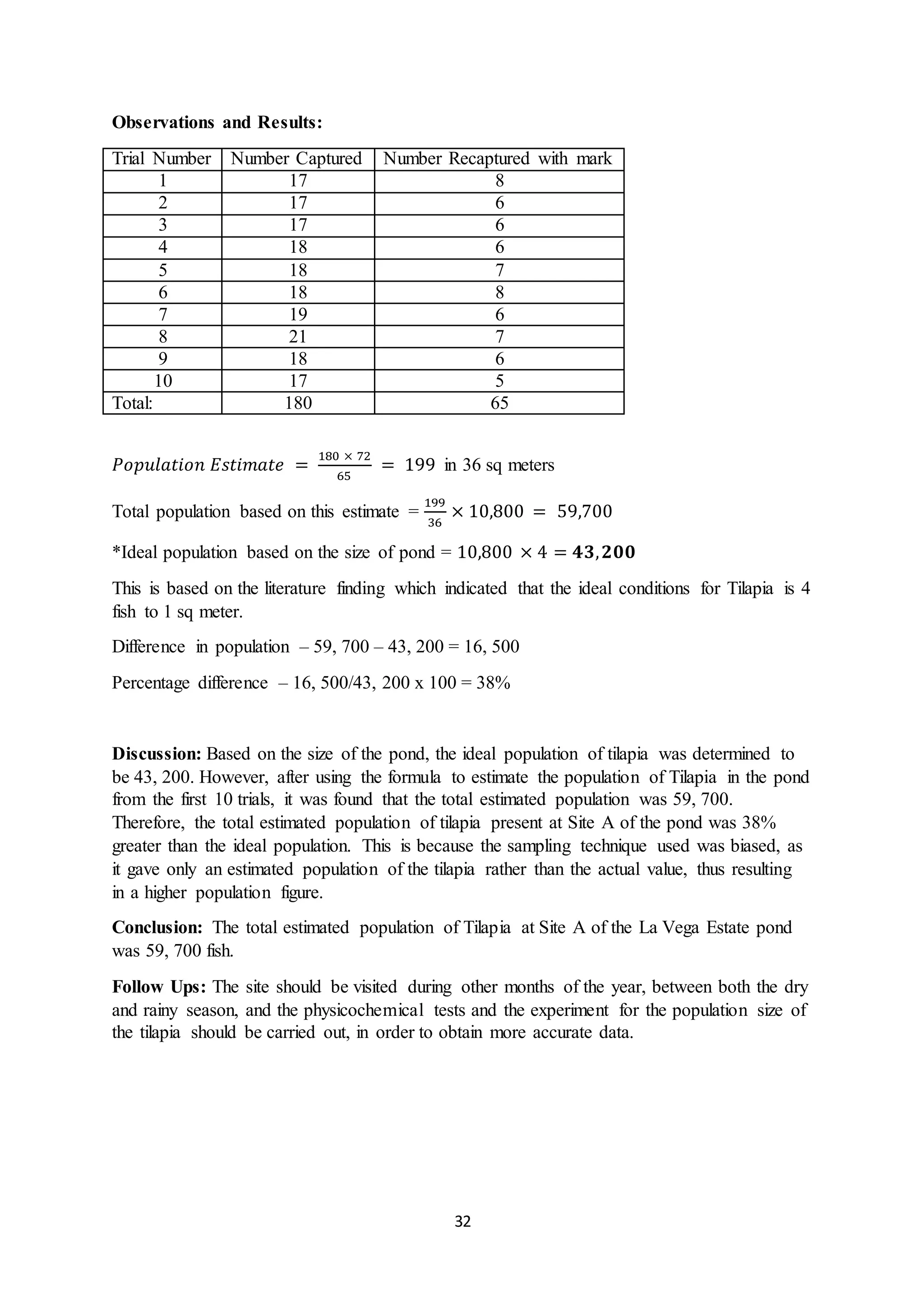
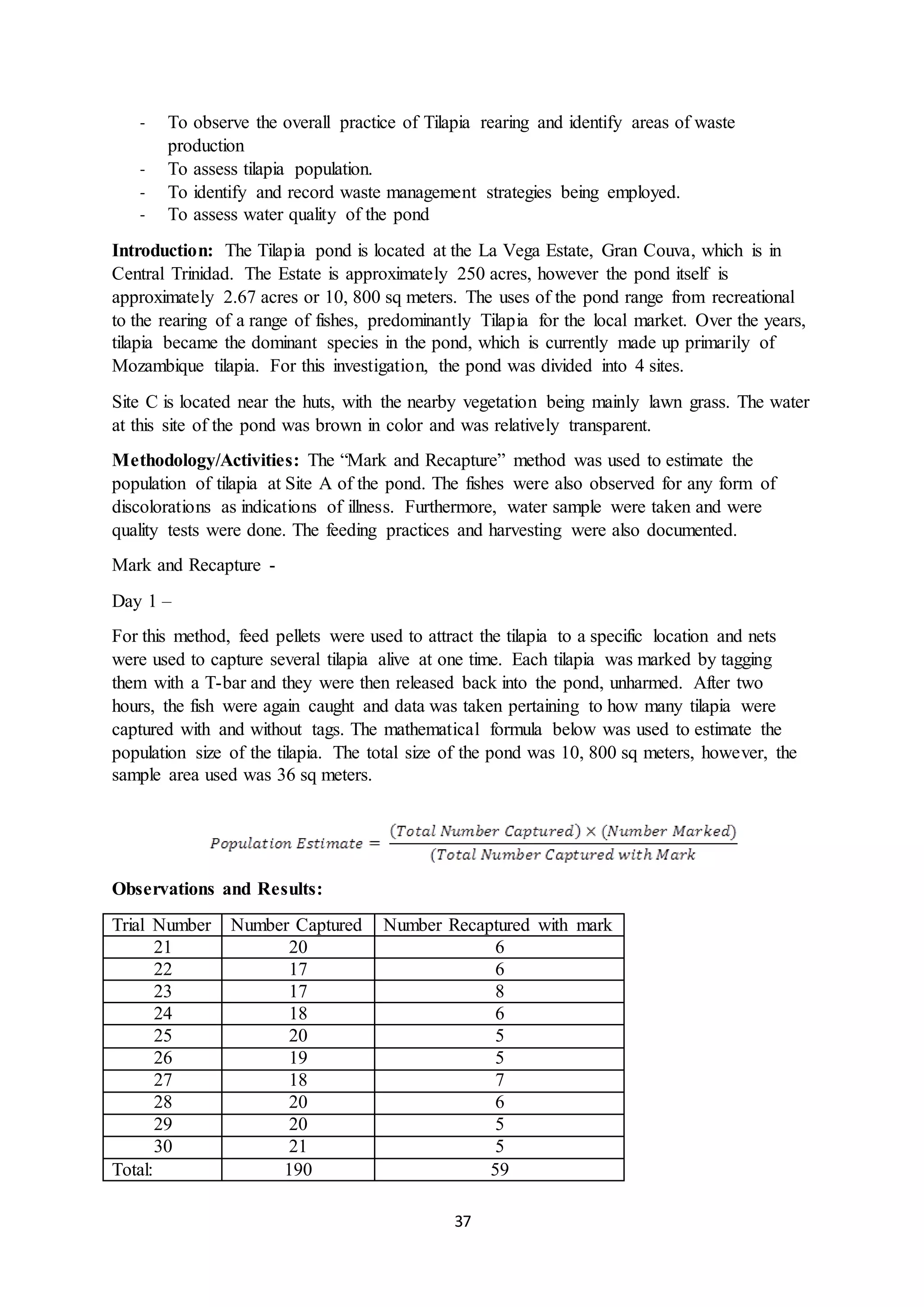
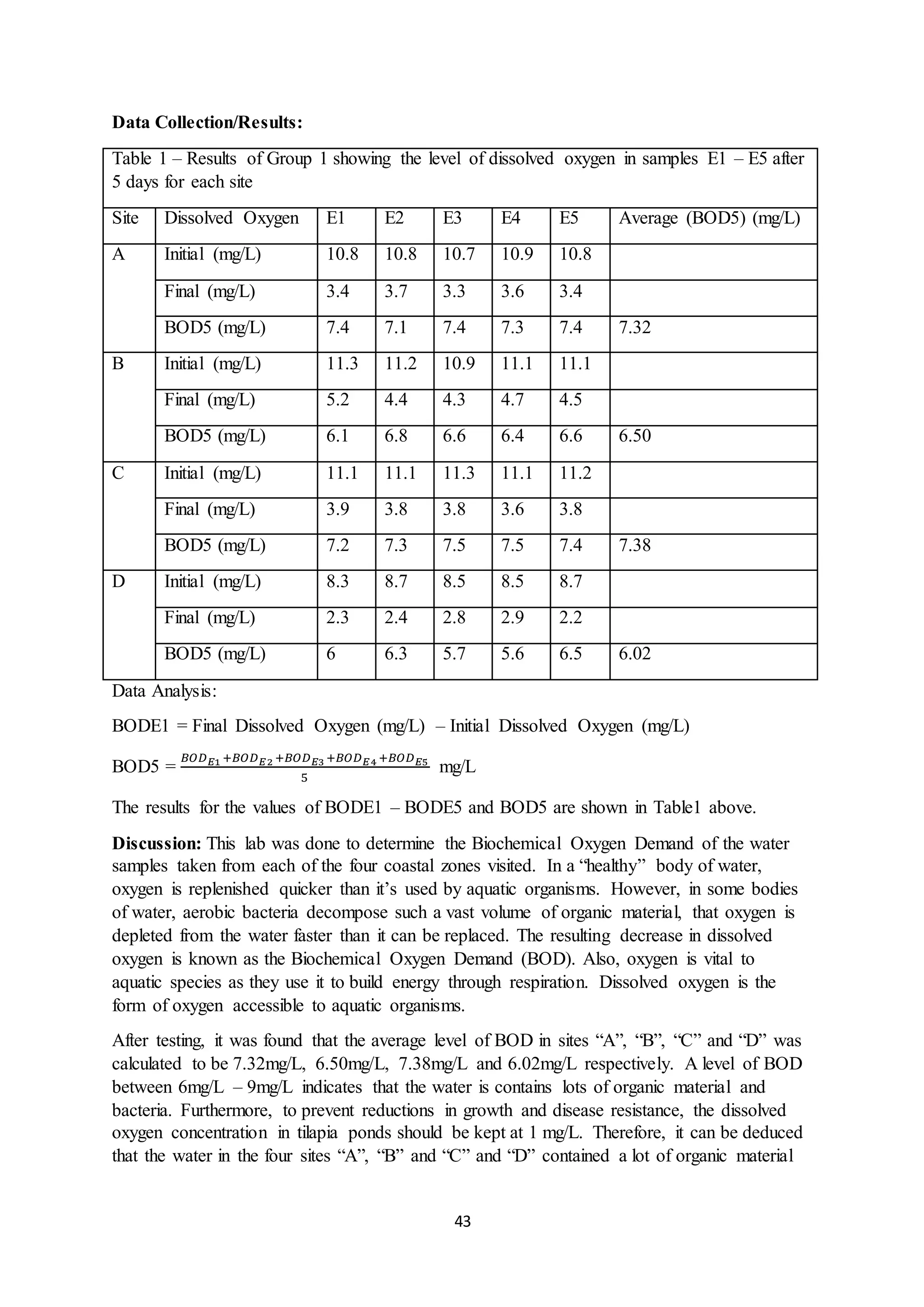
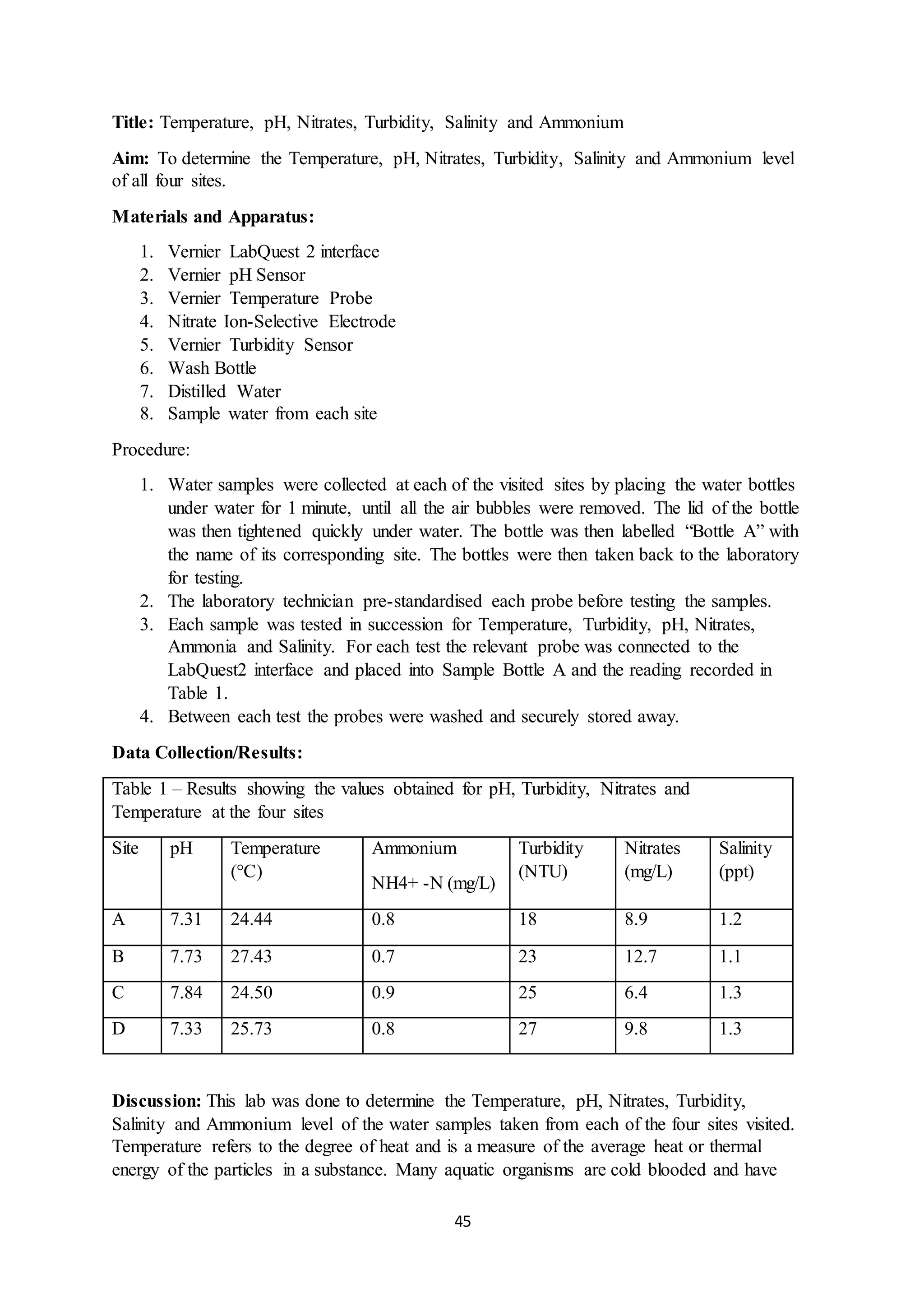
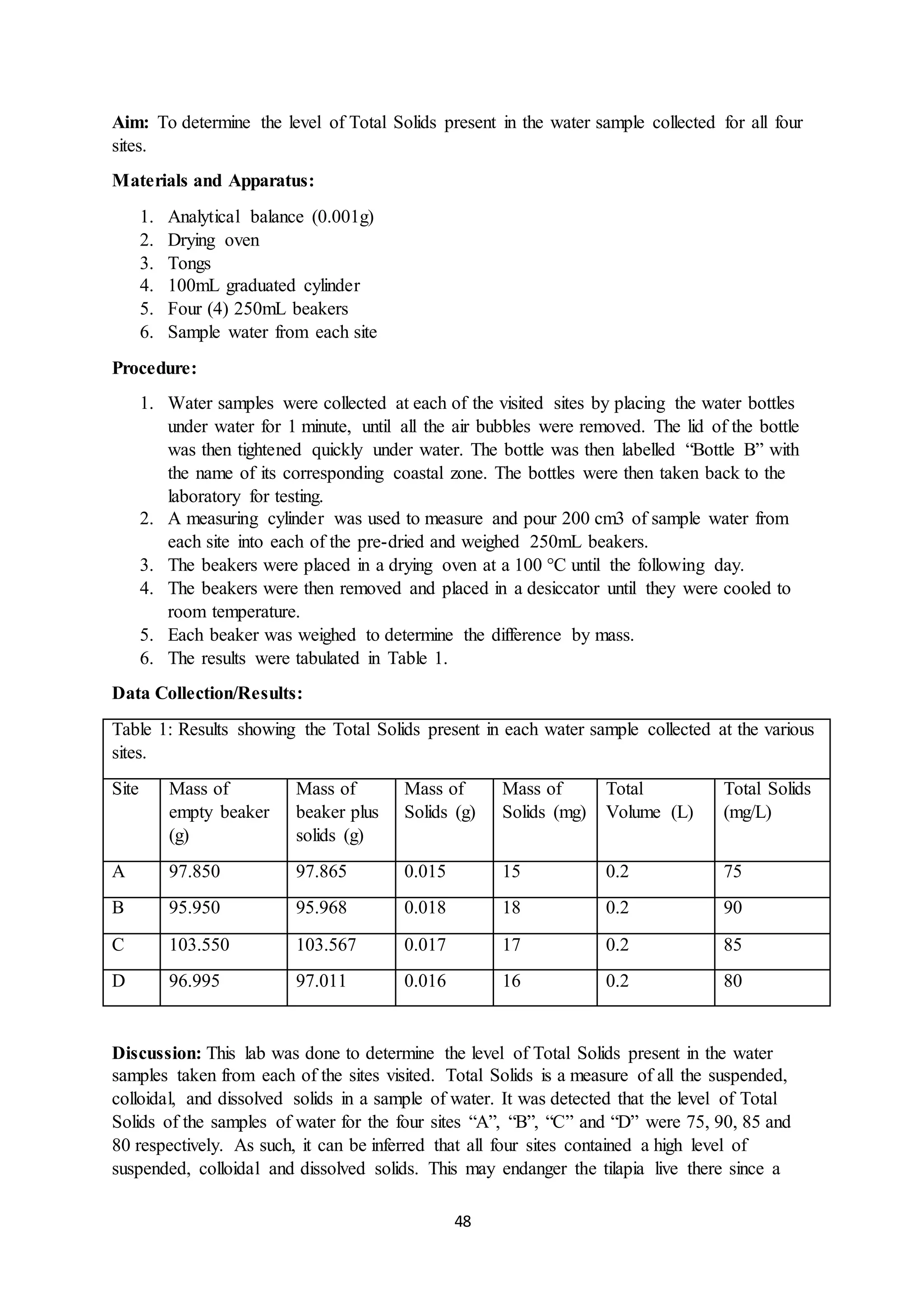
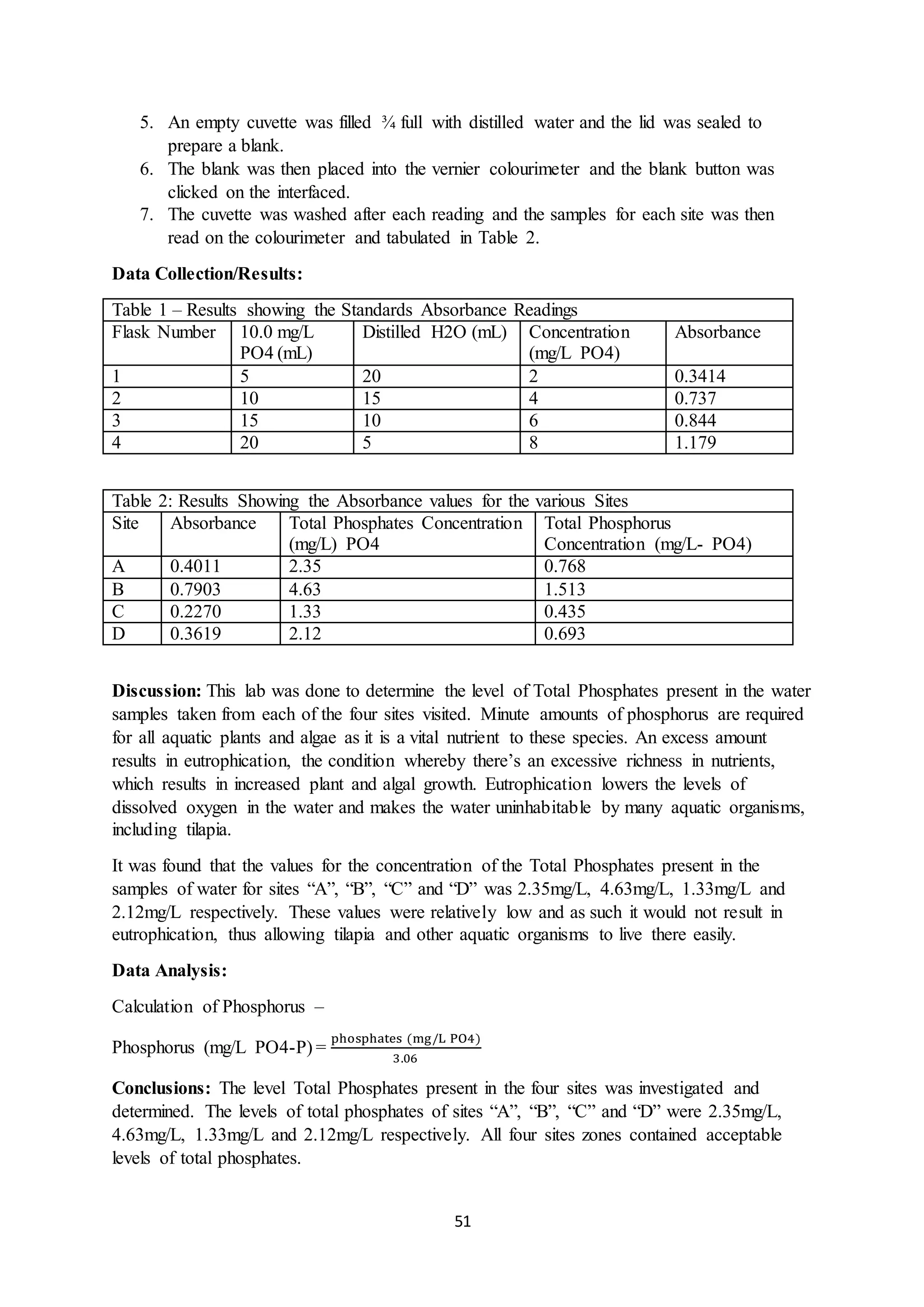
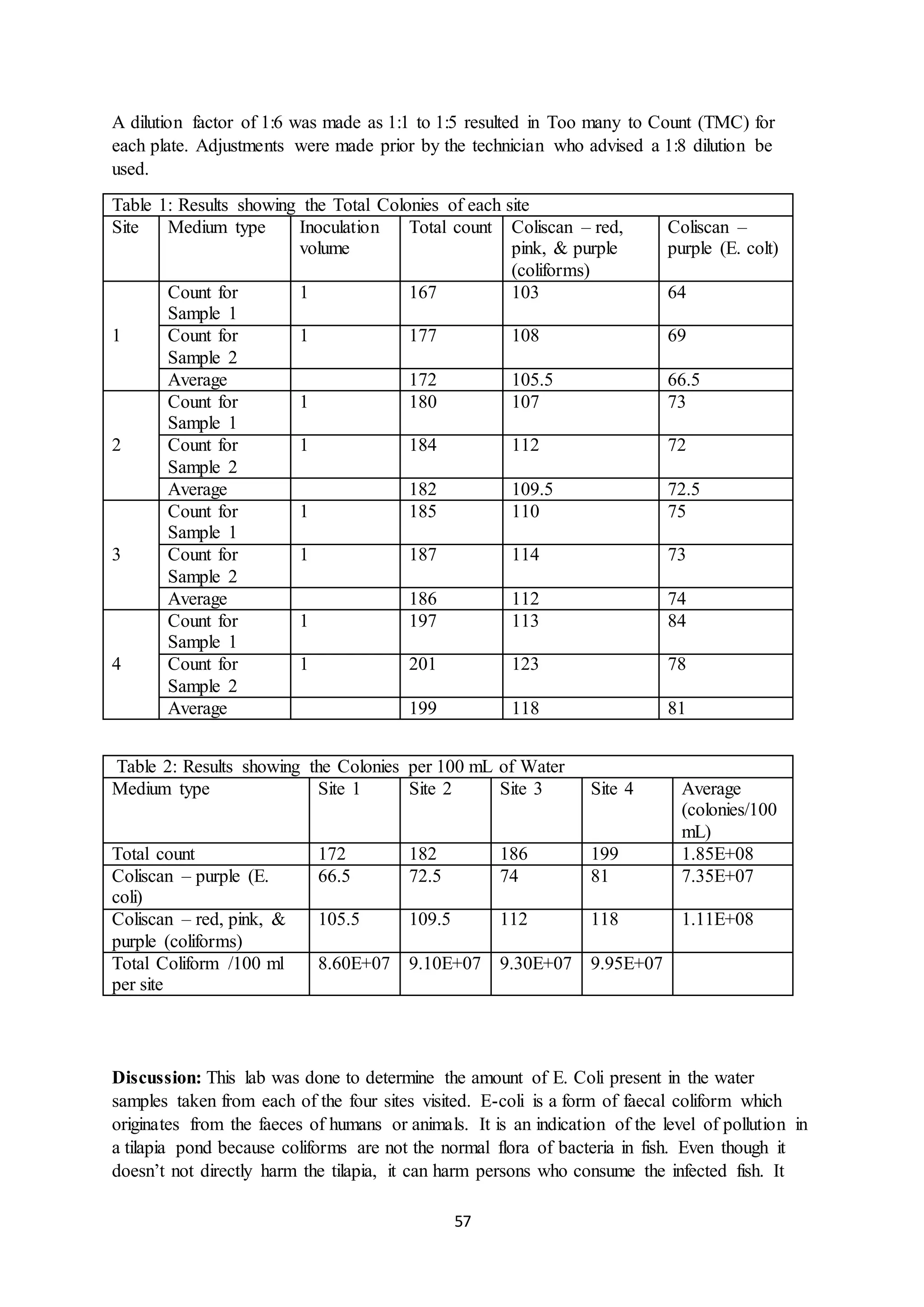
This document is a student's internal assessment report on the rearing practices of tilapia in a pond in Trinidad. It includes an introduction describing tilapia and its commercialization in Trinidad. The literature review discusses tilapia rearing methods, feeding, impacts on water quality, and various water quality tests. The report then describes the student's methodology, laboratory tests conducted, results, discussions, conclusions and recommendations regarding the effects of tilapia farming on water quality and the environment.