The document discusses the management of pancreatic cancer. Some key points:
- Pancreatic cancer is the 4th leading cause of cancer death. 80% present with metastatic disease.
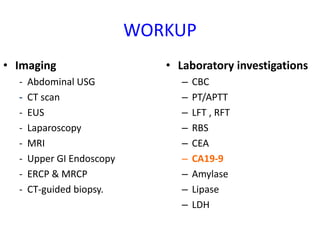
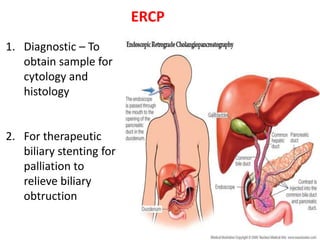
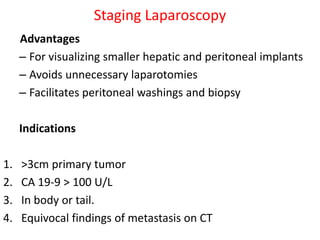
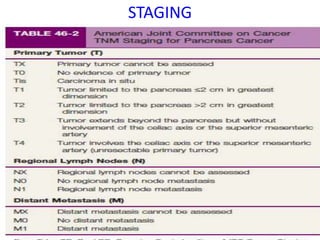
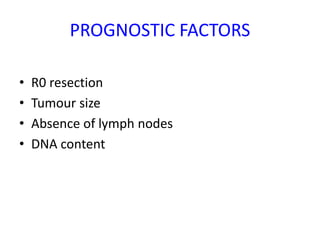
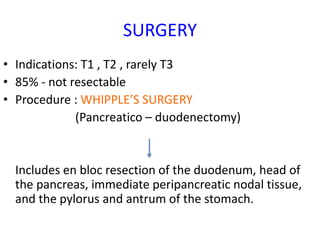
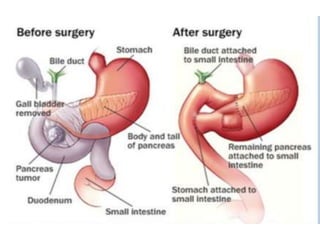
- Diagnosis involves imaging like CT, MRI, EUS and biopsies. Surgery offers the only chance for cure but only 15-20% are resectable.
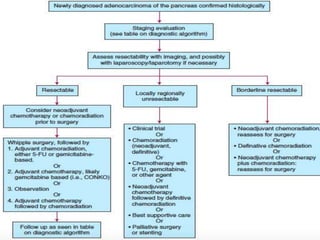
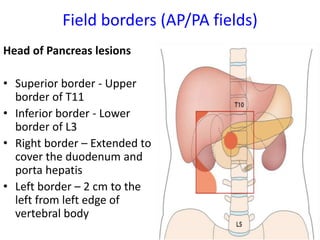
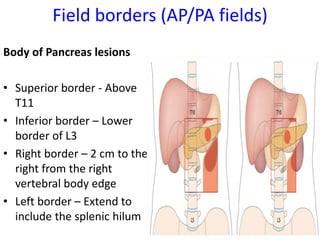
- Treatment depends on resectability - surgery for resectable tumors, chemotherapy and radiation for borderline/advanced cases.
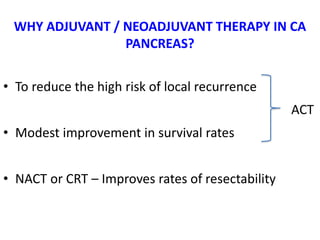
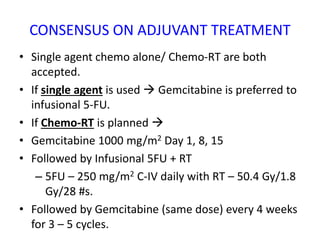
- Adjuvant chemo or chemo-radiation after surgery may improve survival. More aggressive FOLFIRINOX regimen shows better outcomes than gemcitabine alone.
- Molecular targeted therapies and