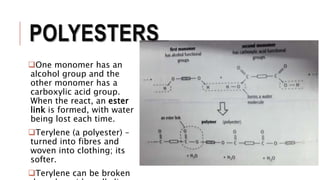
The document discusses the formation and refining processes of fossil fuels, specifically petroleum, highlighting its non-renewable nature and the geological transformations involved. It explains fractional distillation, catalytic cracking, and the role of different fractions in producing fuels like gasoline and diesel, as well as synthetic polymers made from monomers through polymerization. Additionally, it mentions alternative fuels and various types of polymers, detailing their structures and applications in everyday products.