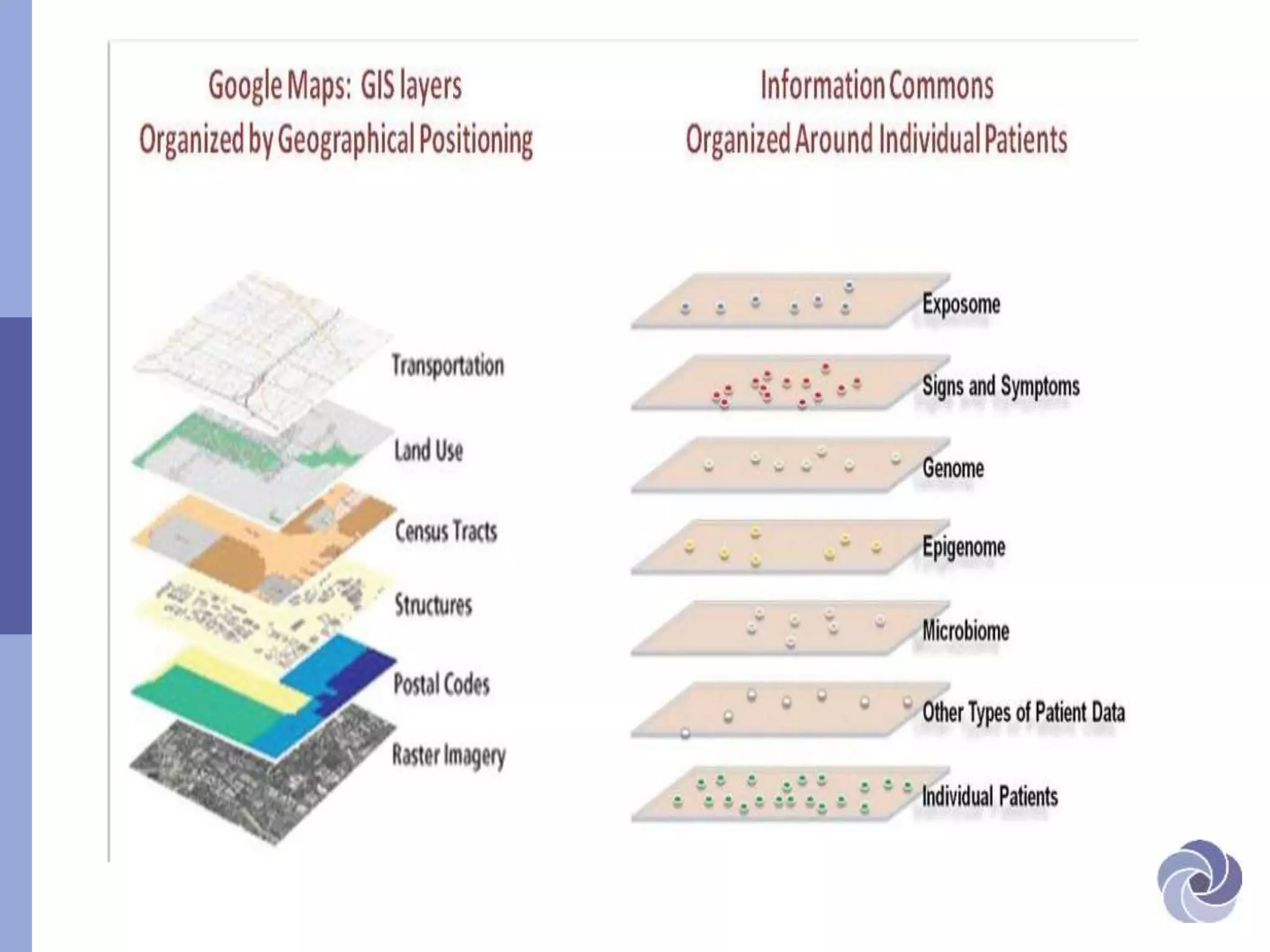
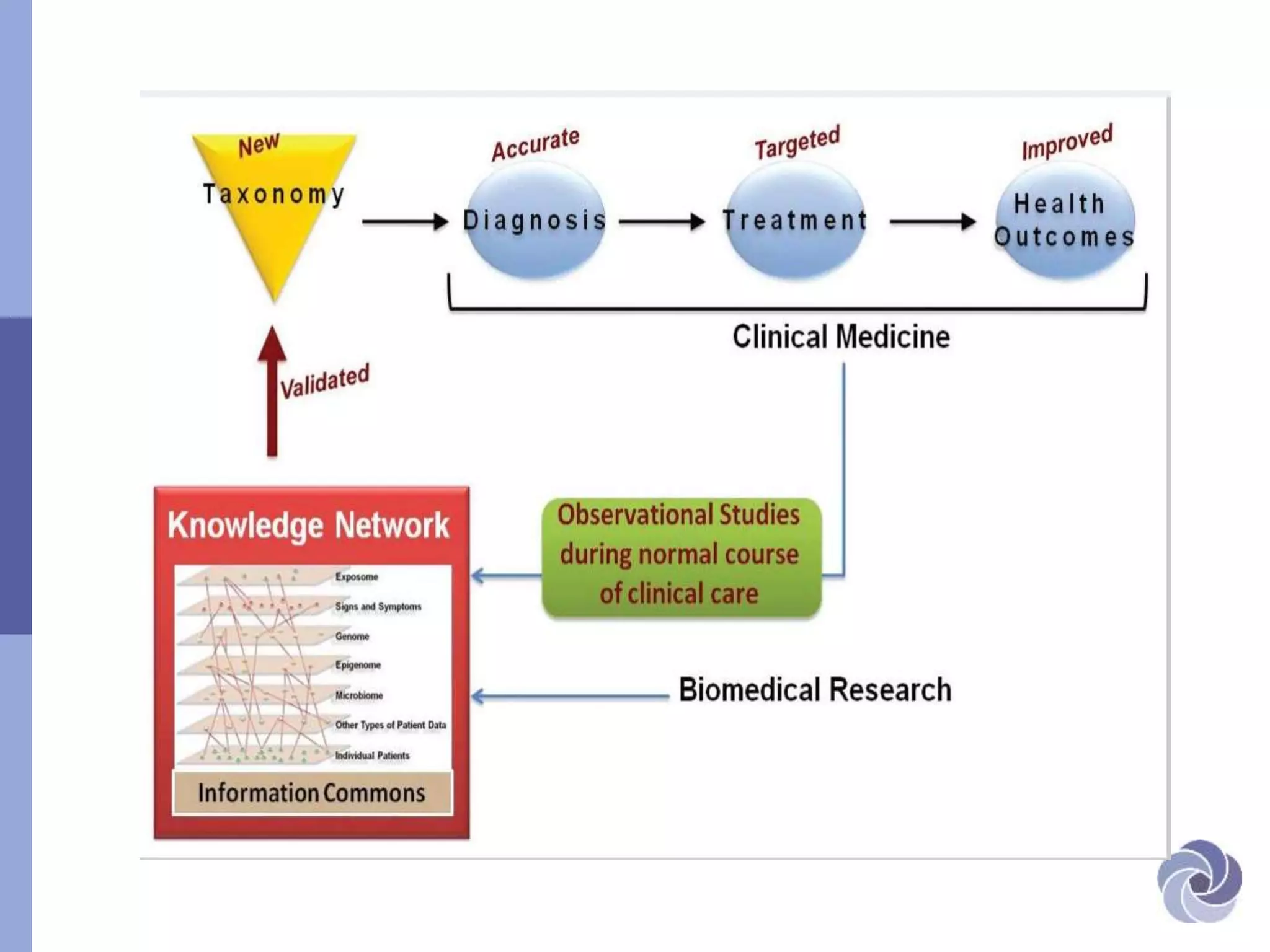
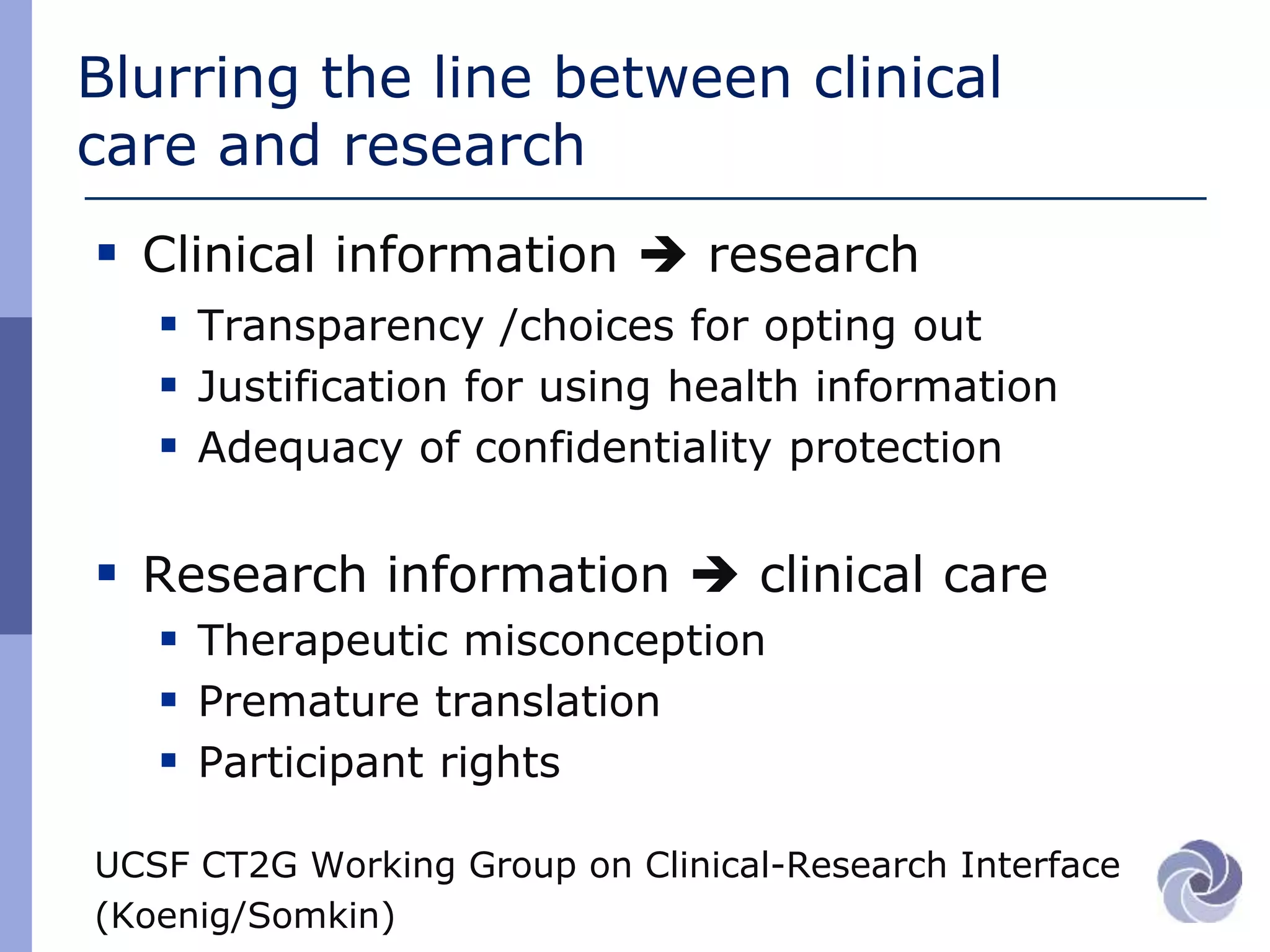
This document discusses several bioethical issues related to genomics and electronic health records. It touches on the challenges of linking disparate health datasets while protecting patient privacy. It also examines the blurring lines between clinical care and research when using individuals' health information. Specifically, it raises questions around informed consent, transparency, justification for using data, and ensuring adequate confidentiality. The document also explores issues of trust in different contexts and public concerns about the use of newborn screening samples in research. Finally, it discusses developing policy around "information commons" and engaging stakeholders to help shape biorepository research standards.

![The challenge…ethically and
scientifically
“…big data becomes transformative when
disparate datasets can be linked at the
individual person level… [BUT]
big biomedical data are scattered
across institutions and intentionally
isolated to protect patient privacy.”
Weber et al JAMA 2014](https://image.slidesharecdn.com/burkeucsfinformaticsjune2014-140623150542-phpapp01/75/UCSF-Informatics-Day-2014-Wylie-Burke-Bioethical-Issues-in-Genomics-and-Electronic-Health-Records-2-2048.jpg)