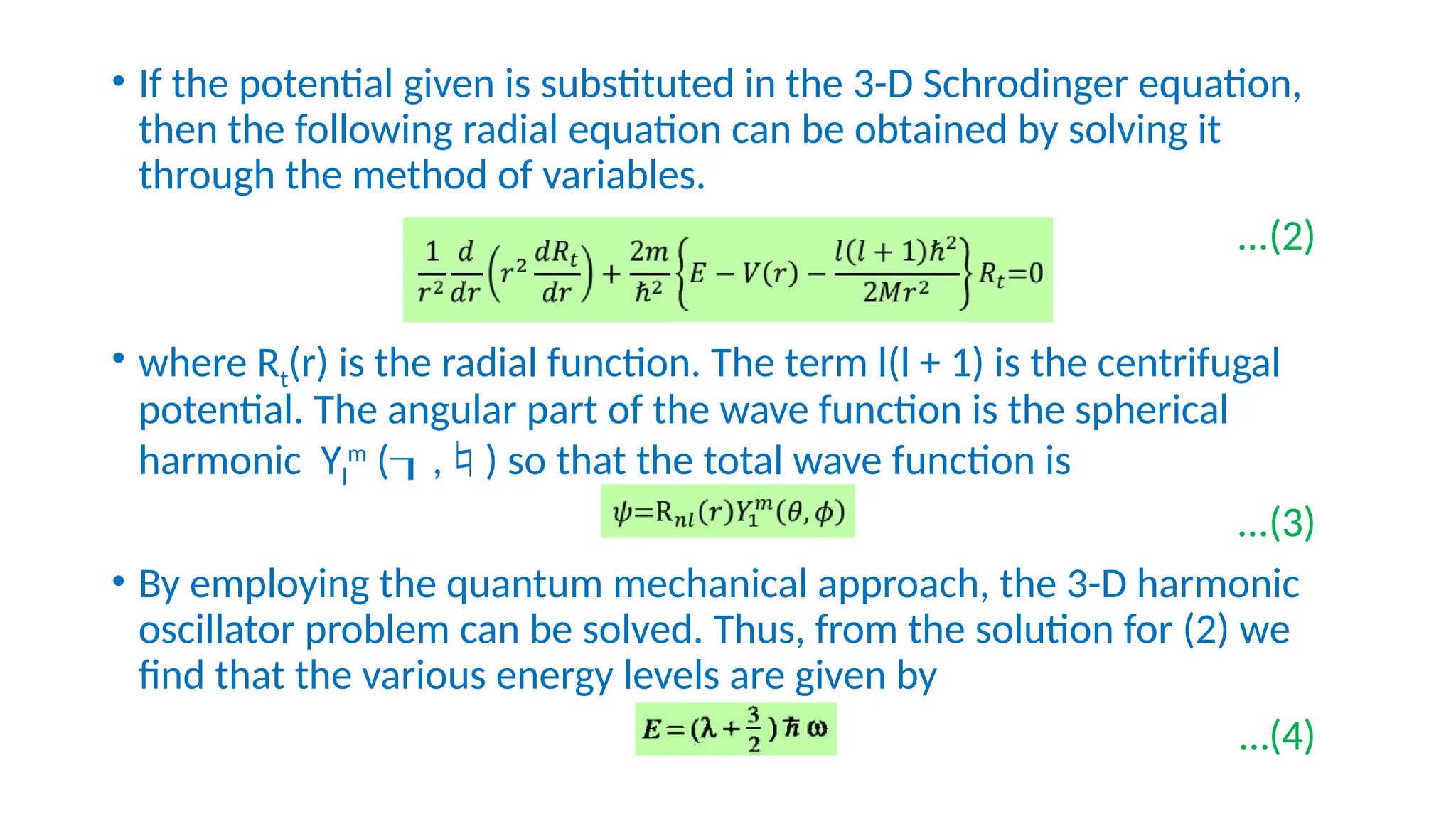
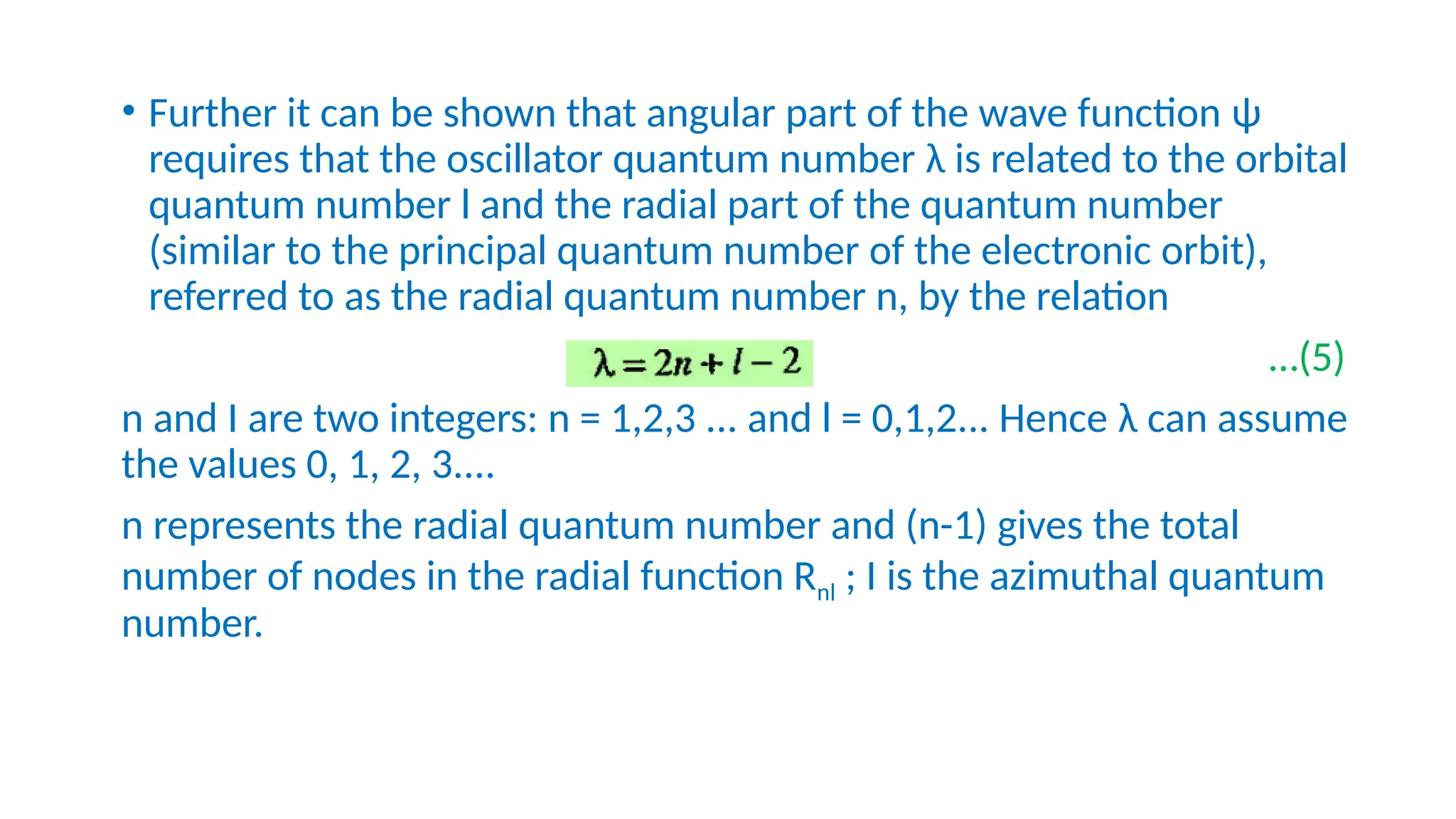
The nuclear shell model describes the arrangement of protons and neutrons in shells, similar to electron configurations, and explains nuclear stability through magic numbers. It highlights how nuclei with certain arrangements exhibit greater stability and abundance, supported by evidence from isotopic ratios and binding energy measurements. The model also employs quantum mechanical principles to derive energy levels and degeneracies of nucleonic states.