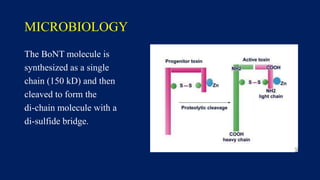
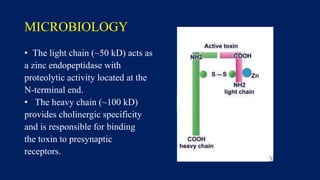
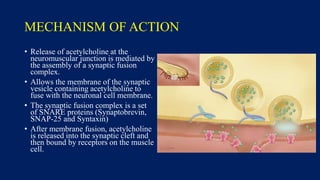
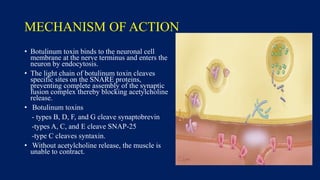
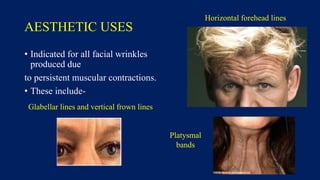
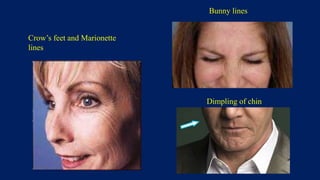
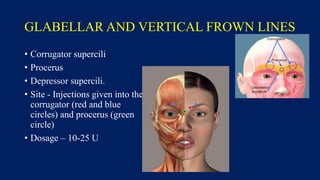
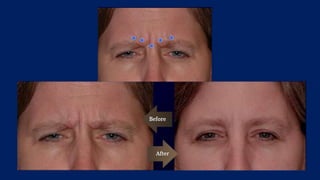
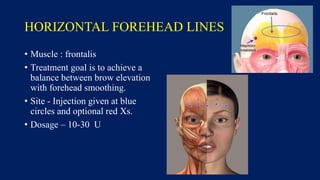
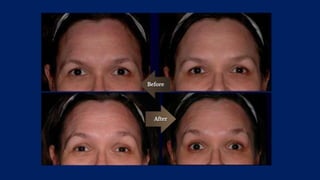
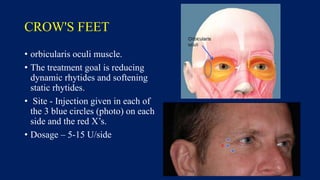
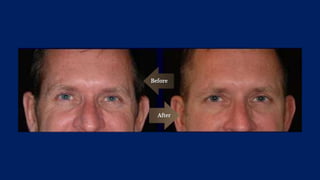
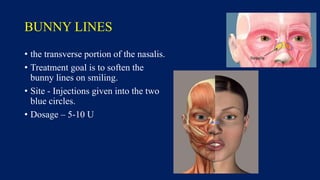
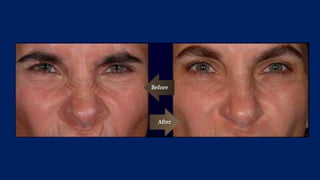
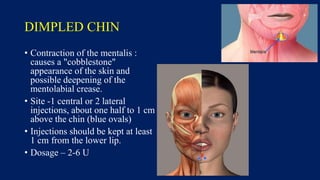
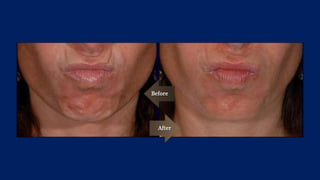
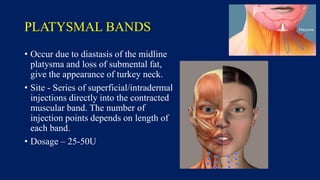
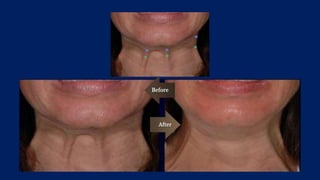
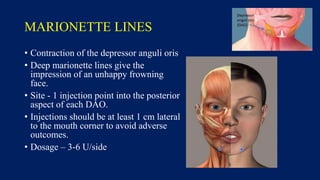
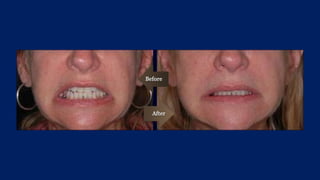
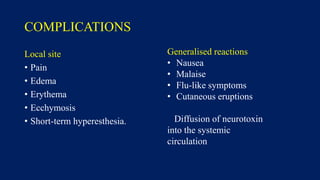
Botulinum toxin is produced by Clostridium botulinum bacteria. It works by blocking the release of acetylcholine at neuromuscular junctions, preventing muscle contraction. It has been used since the 1980s to treat medical conditions involving muscle overactivity like strabismus and dystonia. In the 1990s, its use was explored for cosmetic purposes to reduce facial wrinkles. The FDA approved its use for frown lines in 2002 and excessive underarm sweating in 2004. It is injected into specific facial muscles to weaken them and smooth wrinkles. Common sites include the glabella, forehead, crow's feet, bunny lines, marionette lines and platysmal bands. Potential complications include



![BOTULINUM TOXIN (BTX OR BoNT)
• Produced by Clostridium botulinum, a gram-positive anaerobic, spore
forming bacillus.
• BoNT is broken into 7 neurotoxins (labeled as types A, B, C [C1, C2],
D, E, F, and G), which are antigenically and serologically distinct but
structurally similar.
• Type A is the most potent toxin, followed by types B and F toxin.
• Human botulism is caused mainly by types A, B, E, and (rarely) F.
Types C and D cause toxicity only in animals.](https://image.slidesharecdn.com/botulinumtoxininaesthesis-210125134230/85/BOTOX-Botulinum-toxin-in-aesthesis-4-320.jpg)