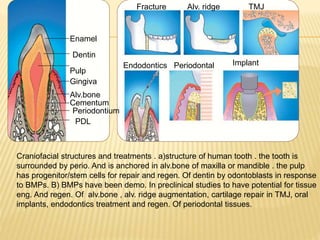
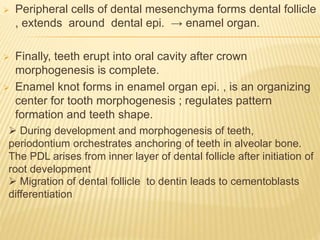
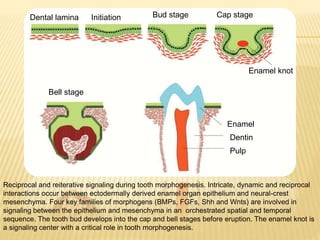
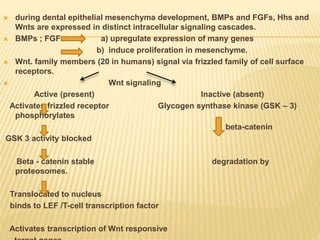
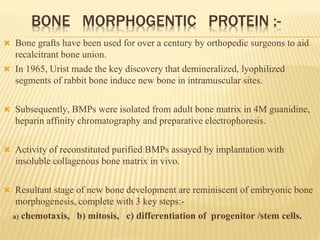
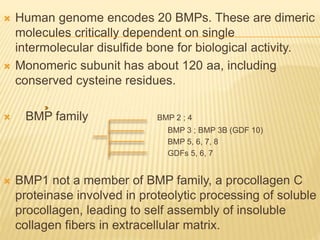
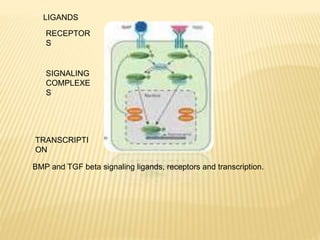
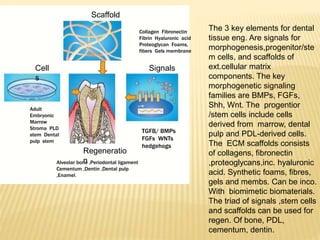
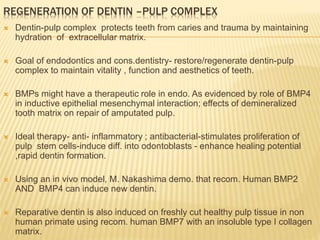
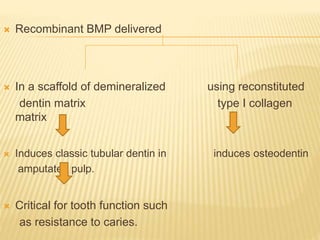
1) BMPs play an important role in tooth development and regeneration by signaling between dental epithelium and mesenchyme. They are expressed in a spatial and temporal sequence that governs tooth patterning and morphogenesis.
2) Progenitor/stem cells found in dental pulp and periodontal ligament have the potential to differentiate into odontoblasts and other dental tissues when exposed to BMPs.
3) The application of BMPs, stem cells, and appropriate extracellular matrix scaffolds could facilitate the regeneration of dental and craniofacial tissues through recapitulating embryonic development pathways.



![ Tissue engineering offers a new option to supplement
existing treatment regimes for periodontal disease.
Potential of regenerative treatment in this fields will require
integration of 3 keys elements :-
a) inductive morphogenetic signals
b) responding progenitor / stem cells
c) extracellular matrix scaffold
These elements must be combined to facilitate a
development cascade of pattern formation ; craniofacial
plan establishment and creation of mirror – image bilateral
symmetry of teeth in maxilla and mandible.[ oral cavity offers
distinct advantages such as ease of observation and accessibility].
It will also require a recapitulation of some of the
mechanism of embryonic development and morphogenesis.](https://image.slidesharecdn.com/bonemorphogenticprotein-150920081956-lva1-app6891/85/Bone-morphogentic-protein-4-320.jpg)