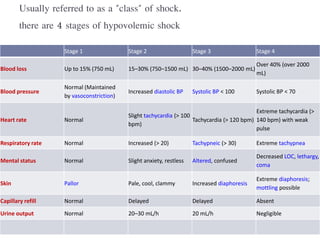
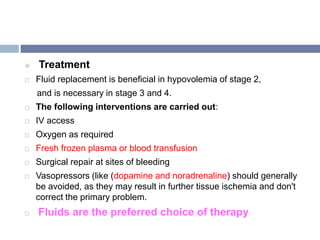
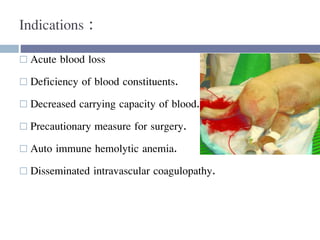
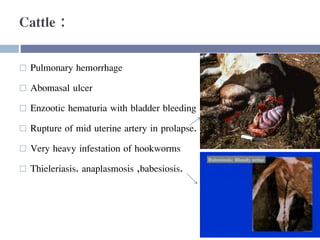
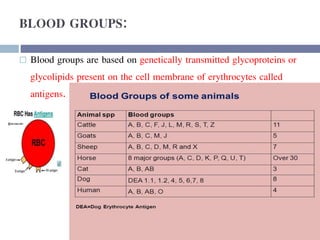
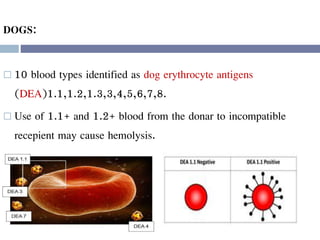
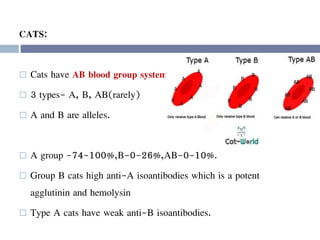
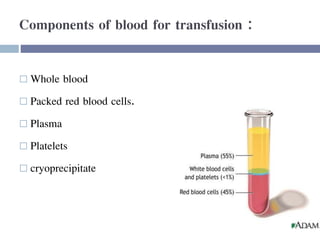
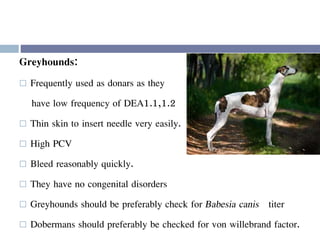
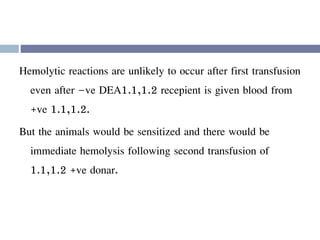
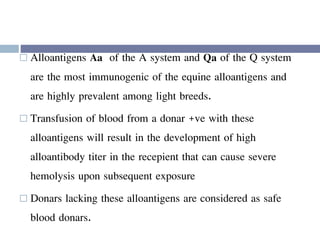
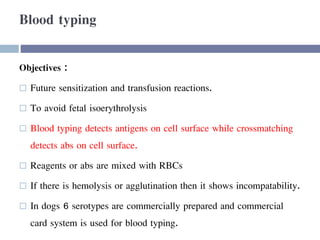
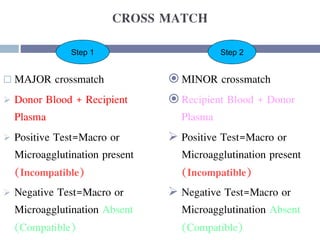
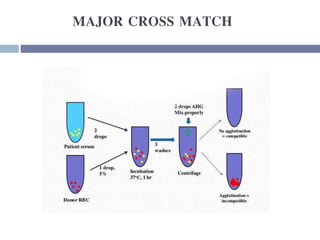
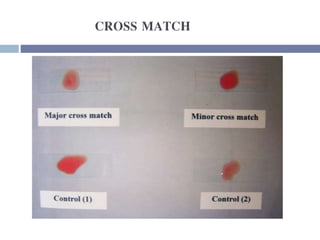
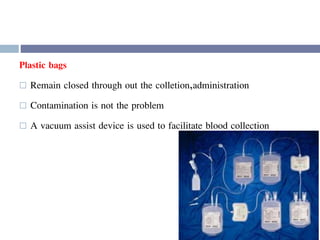
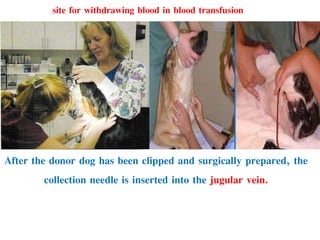
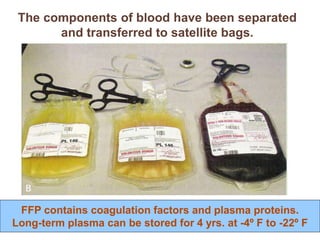
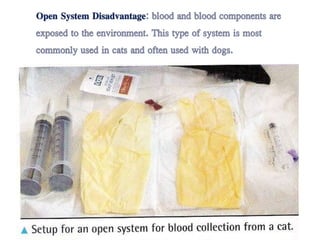
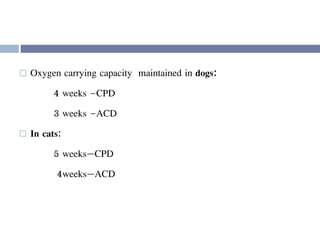
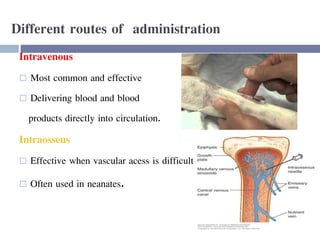
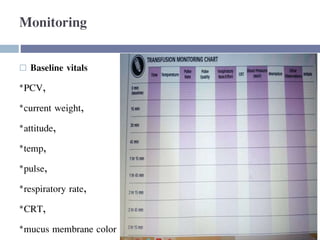
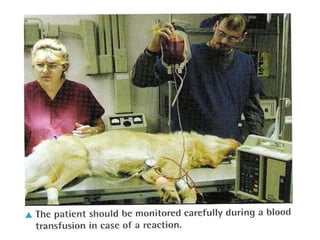
This document discusses hypovolemia, its causes, signs, diagnosis and treatment. It then discusses blood transfusion in animals, including definitions, indications for transfusion, blood groups in different species, components of blood that can be transfused, donor selection criteria, blood typing and cross-matching processes, blood collection and storage methods, administration of transfusions, and monitoring for transfusion reactions. Key points covered include the 4 stages of hypovolemic shock, common causes of hypovolemia, fluid replacement and vasopressors as treatment, and use of whole blood, packed red blood cells, plasma, platelets and cryoprecipitate depending on the indication for transfusion.