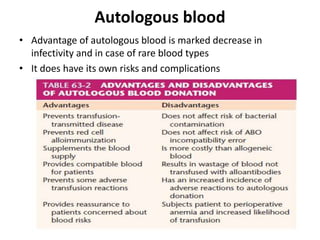
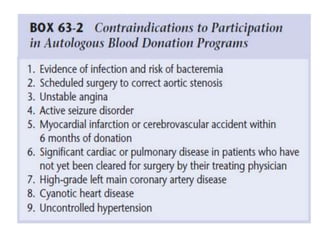
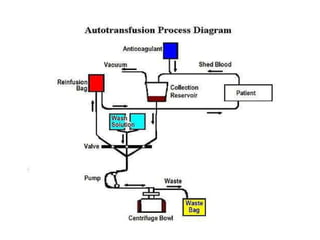
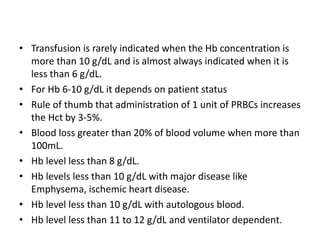
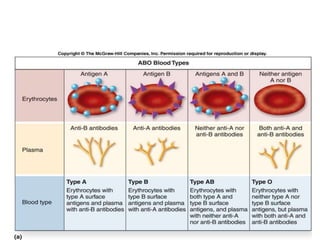
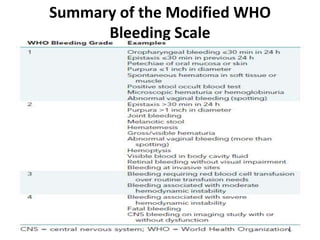
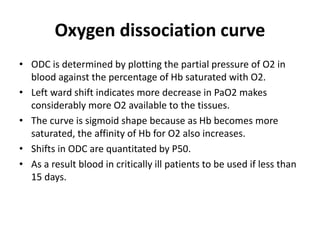
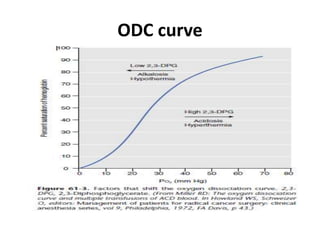
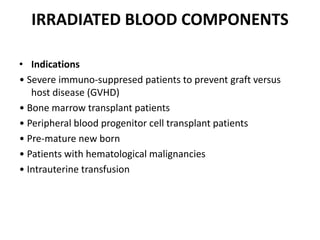
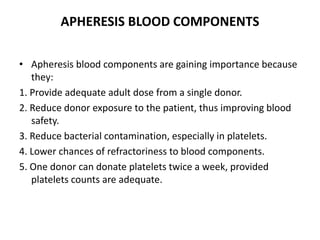
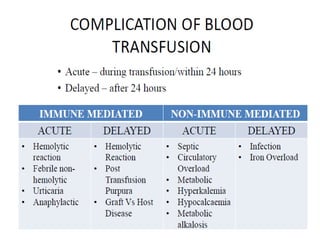
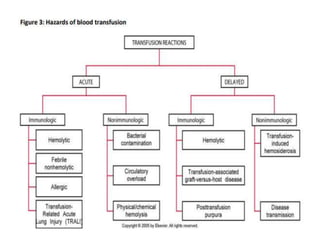
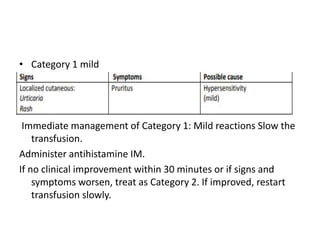
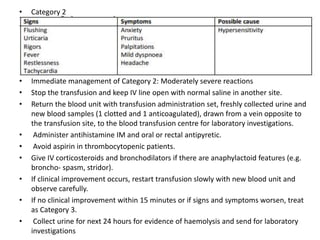
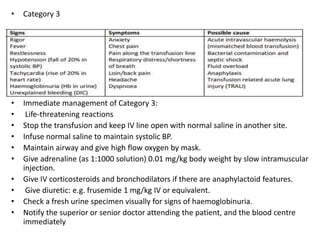
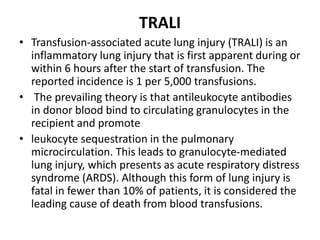
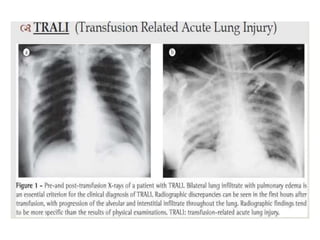
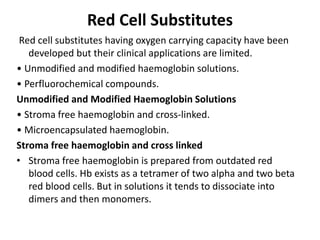
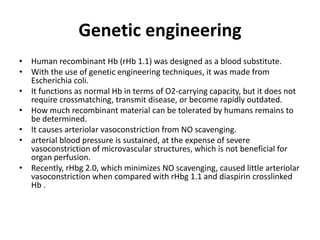
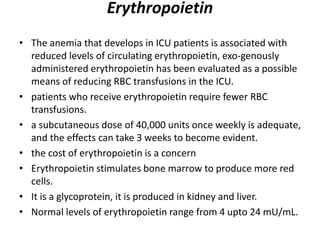
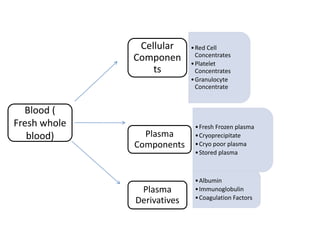
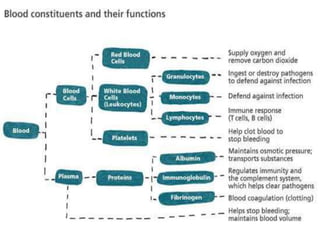
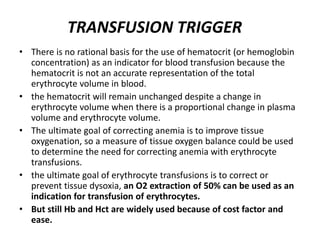
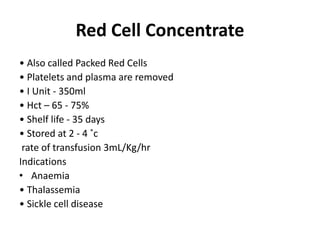
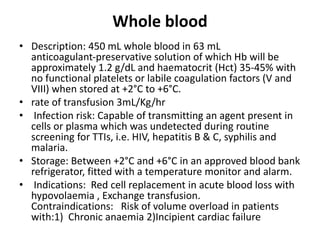
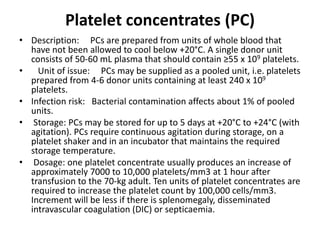
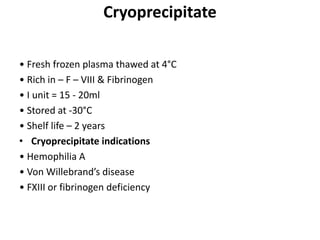
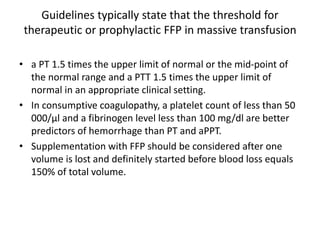
Transfusion of blood and blood products can be used to treat various conditions related to deficiencies in red blood cells, platelets, or clotting factors. There are several types of blood products including packed red blood cells, fresh whole blood, platelet concentrates, fresh frozen plasma, and cryoprecipitate. Massive transfusions involving 10 or more units of blood in 24 hours require special consideration and guidelines recommend maintaining a 1:1:1 ratio of plasma, platelets, and packed red blood cells. Acute normovolemic hemodilution involves removing blood pre-operatively and replacing volume with crystalloids or colloids to reduce transfusion needs during anticipated significant blood loss.







![Massive transfusion
• Massive transfusion is defined as transfusion approximating
or exceeding the patient’s blood volume, or transfusion of
more than 10 units of blood within 24 h.[41]
• Replacement of more than 50% of circulating blood volume
in less than 3 h or transfusion at the rate of more than 150
ml/min is also considered as massive transfusion.
• A blood loss of less then 20% of the total blood volume is
generally well tolerated, while a loss of 20% to 40% will cause
change in the vital signs, with evidence of impaired tissue
perfusion.
• However, loss of more than 40% of blood volume may lead to
frank hemorrhagic shock and progress to circulatory system
failure and cardiac arrest if not corrected.[43]](https://image.slidesharecdn.com/blood-200212063205/85/Blood-16-320.jpg)



![Measurement of blood loss
• Maximum Allowable Blood Loss calculation
EBV calculation: body wt (kg) x average blood volume (ml/kg)
• ABL= [EBV x (Hi-Hf)]/Hi
• EBV=Estimated Blood Volume, Hi= initial hemoglobin, Hf=
final hemoglobin.
• Average blood volumes
• Premature Neonates 95 mL/kg
• Full Term Neonates 85 mL/kg
• Infants 80 mL/kg
• Adult Men 75 mL/kg
• Adult Women 65 mL/kg](https://image.slidesharecdn.com/blood-200212063205/85/Blood-22-320.jpg)



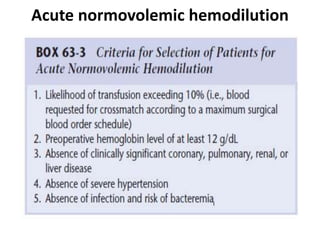
![Acute normovolemic hemodilution
• ANH is the removal of whole blood from a patient while
restoring the circulating blood volume with an acellular fluid
shortly before an anticipated significant surgical blood loss.
• blood is then stored at room temperature and reinfused
during surgery after major blood loss has ceased, or sooner if
indicated.
• Simultaneous infusions of crystalloid (3 mL crystalloid for each
1 mL of blood withdrawn) and colloid (dextrans, starches,
gelatin, albumin [1 mL for each 1 mL of blood withdrawn])
have been recommended.
• Blood units are reinfused in the reverse order of collection
because the first unit collected and therefore the last to be
infused will have the highest hematocrit and concentration of
coagulation factors and platelets.](https://image.slidesharecdn.com/blood-200212063205/85/Blood-27-320.jpg)