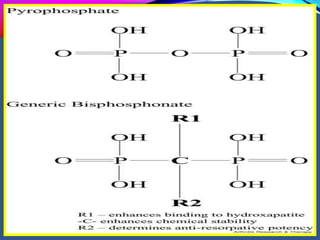
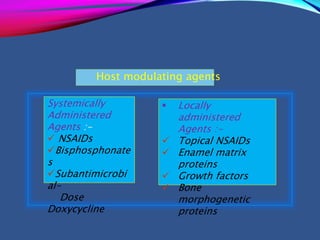
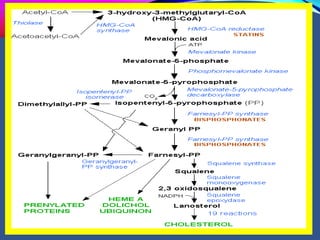
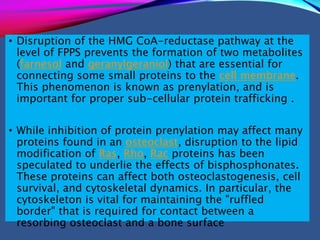
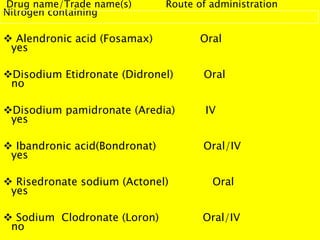
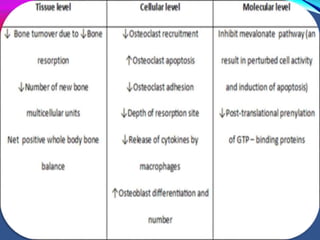
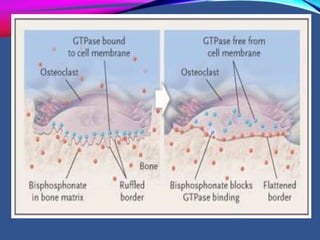
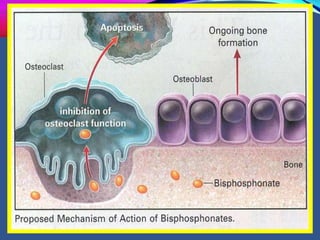
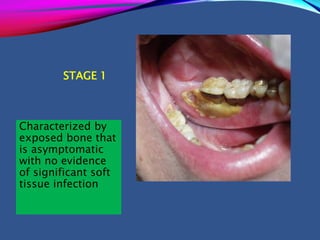
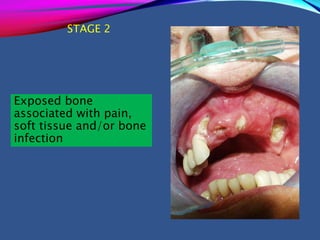
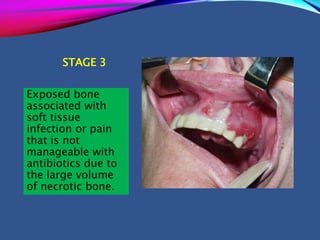
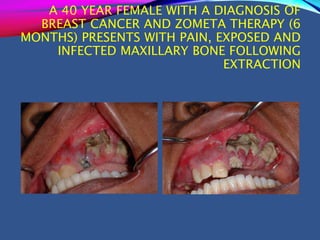
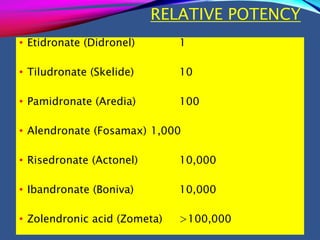
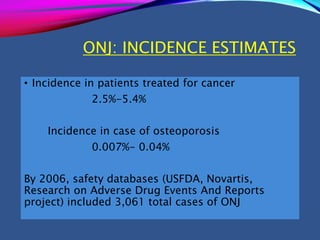
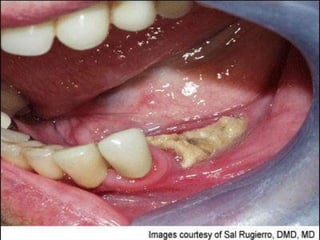
This document provides an overview of bisphosphonates. It discusses their history, structure, indications, mechanisms of action, roles in periodontal therapy, side effects including BRONJ (bisphosphonate-related osteonecrosis of the jaw), and dental management considerations for patients receiving oral bisphosphonate therapy. The document covers bisphosphonates' effects at the tissue, cellular, and molecular levels and reviews both animal and human studies on their use in periodontal applications. It also addresses their potential analgesic properties and optimal duration of use.