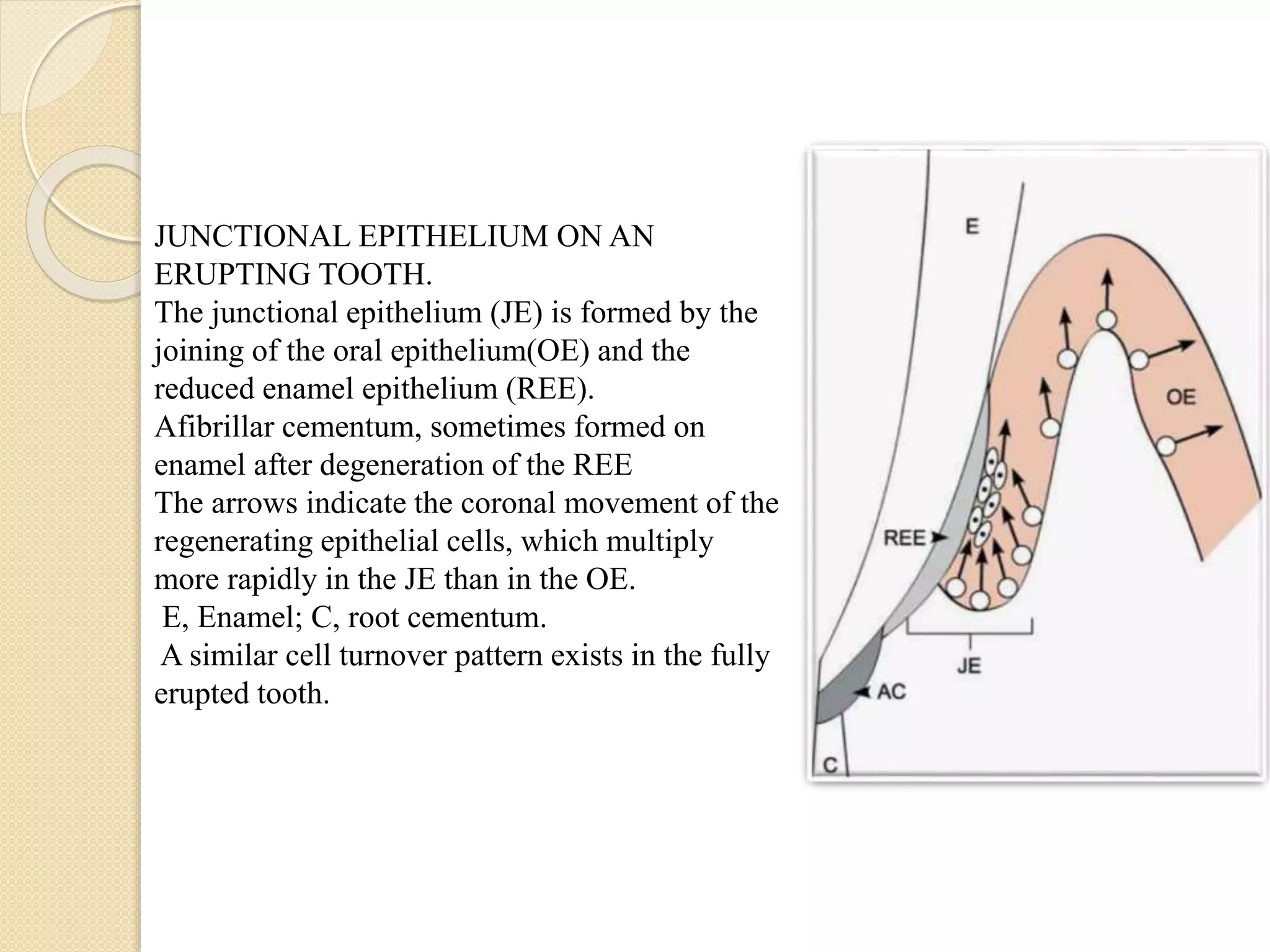
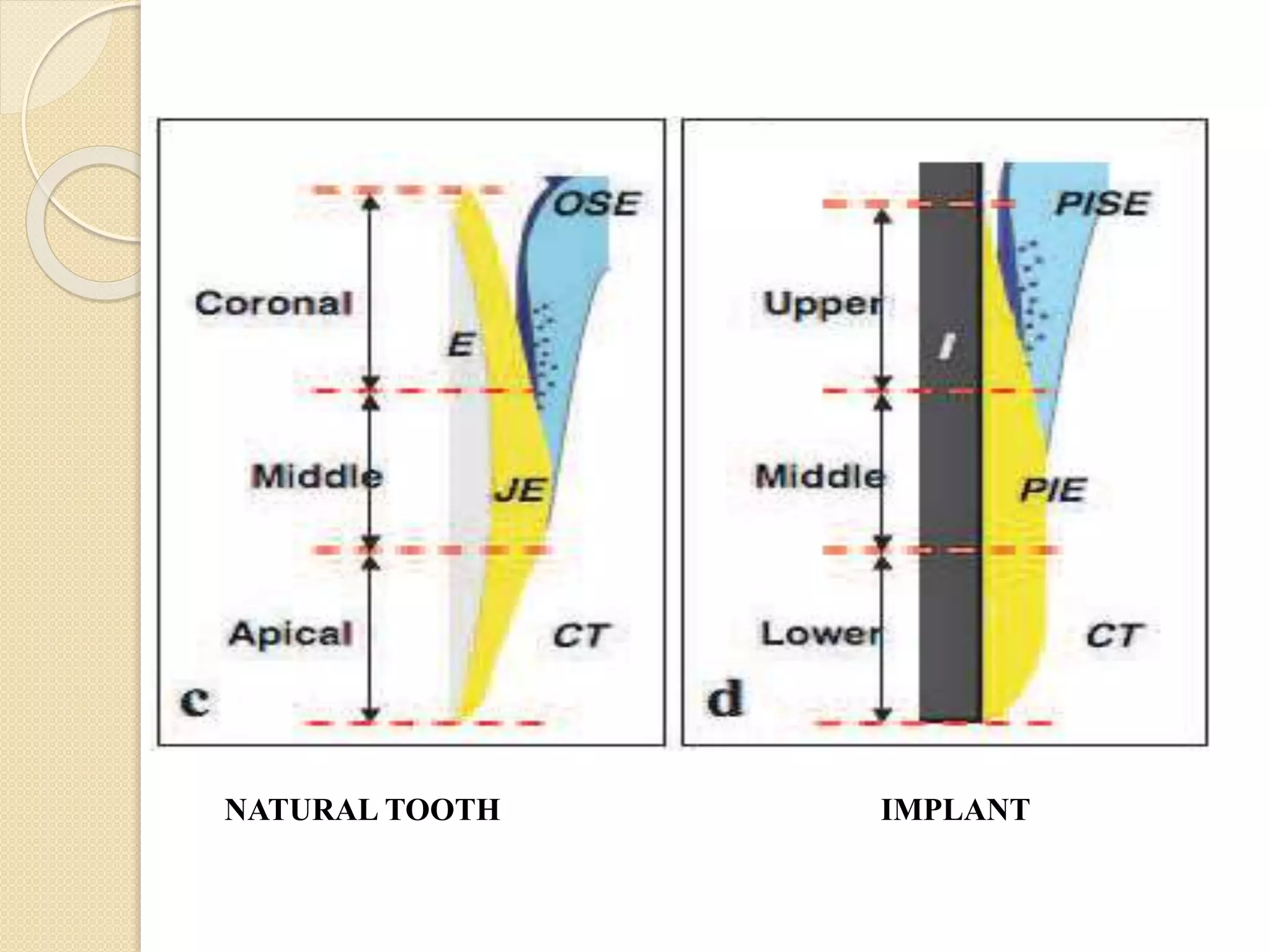
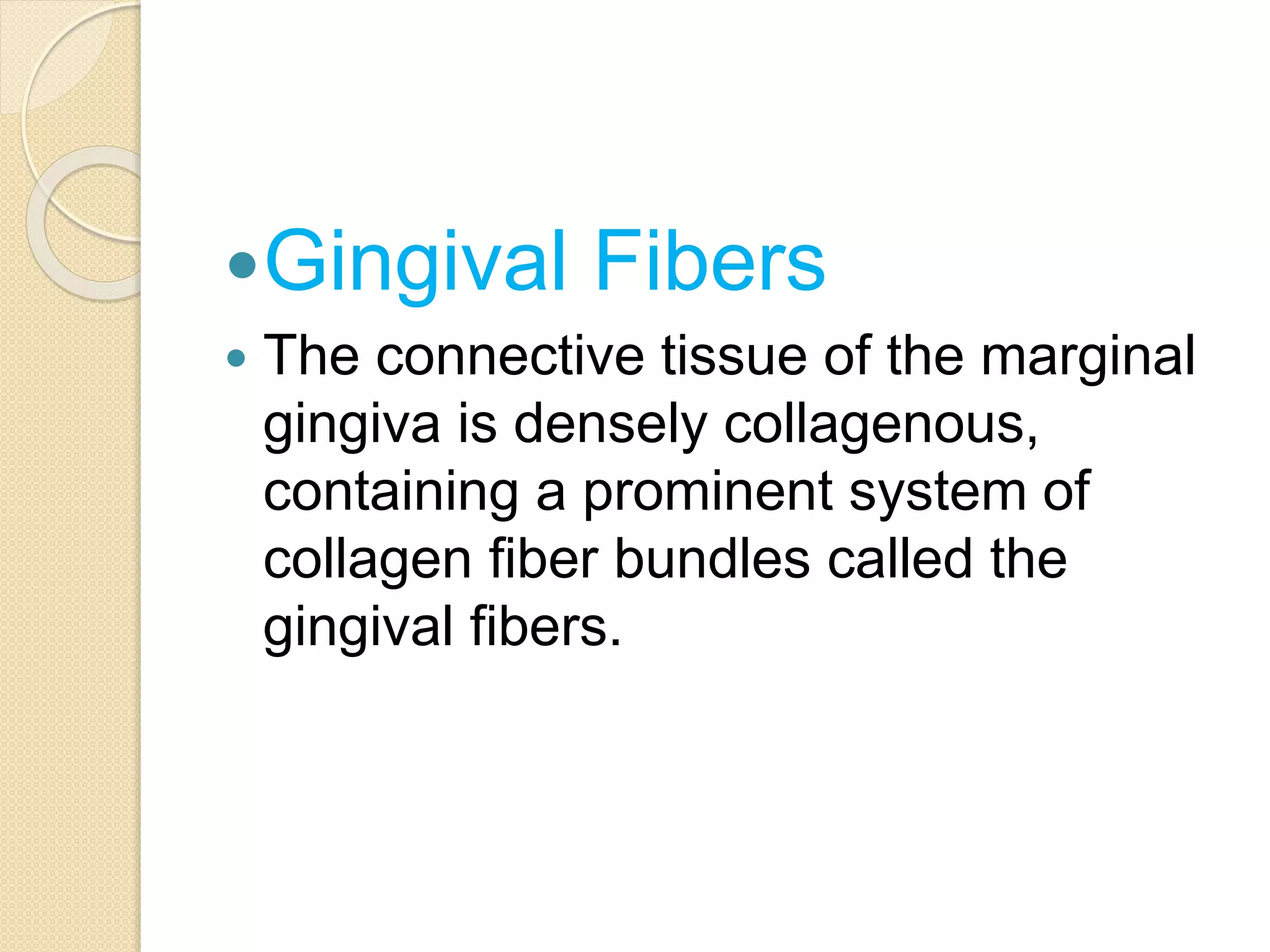
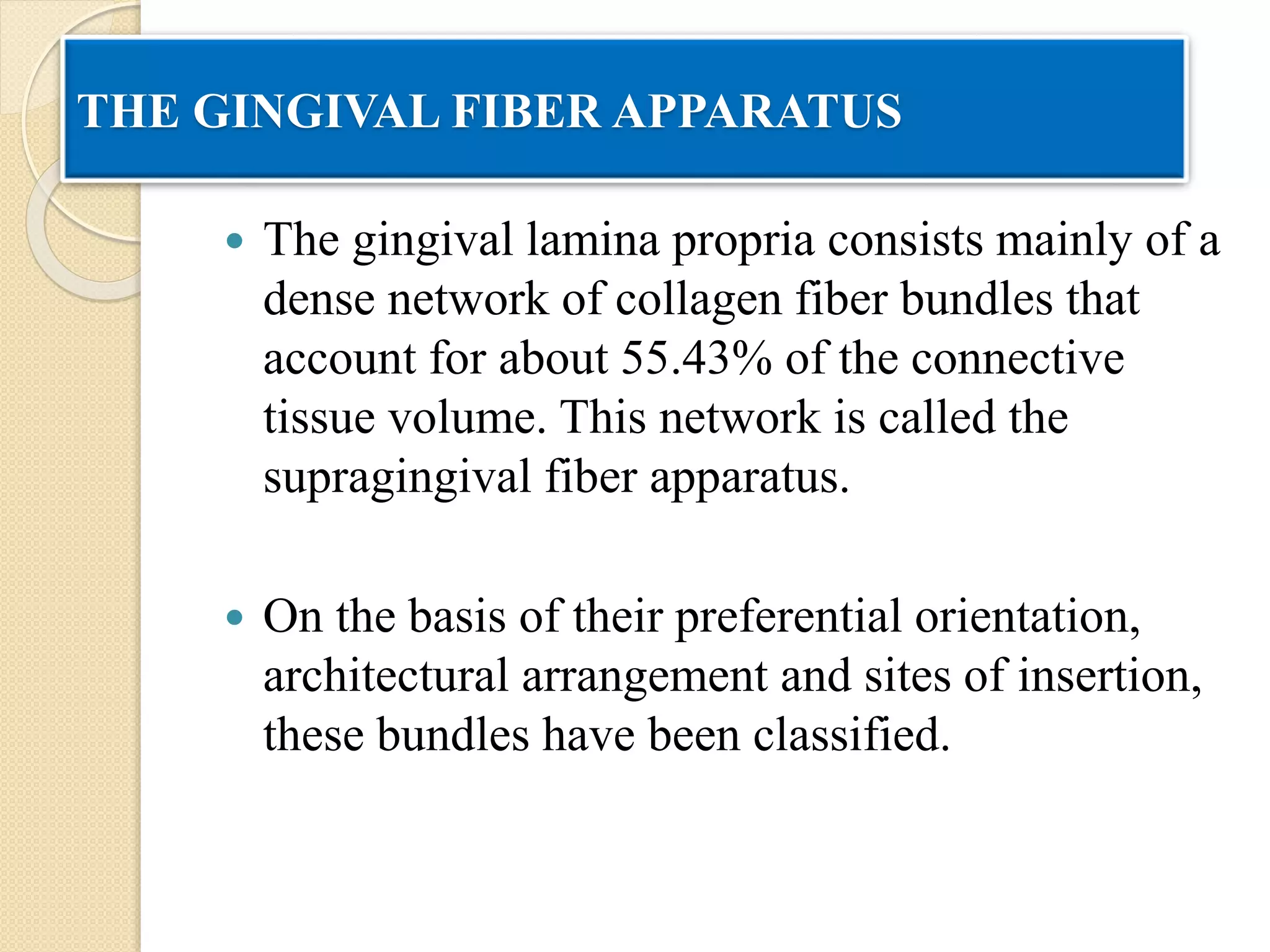
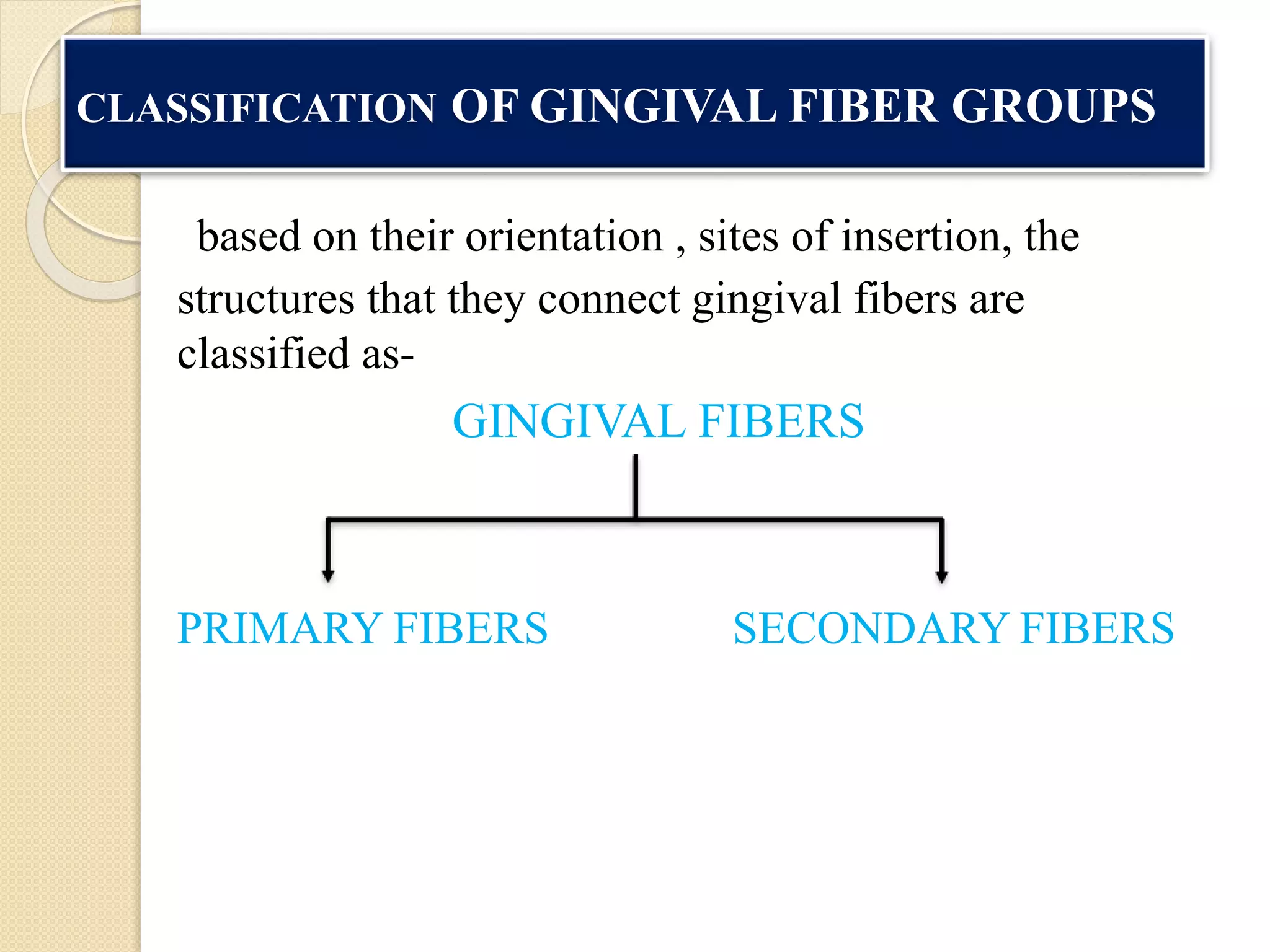
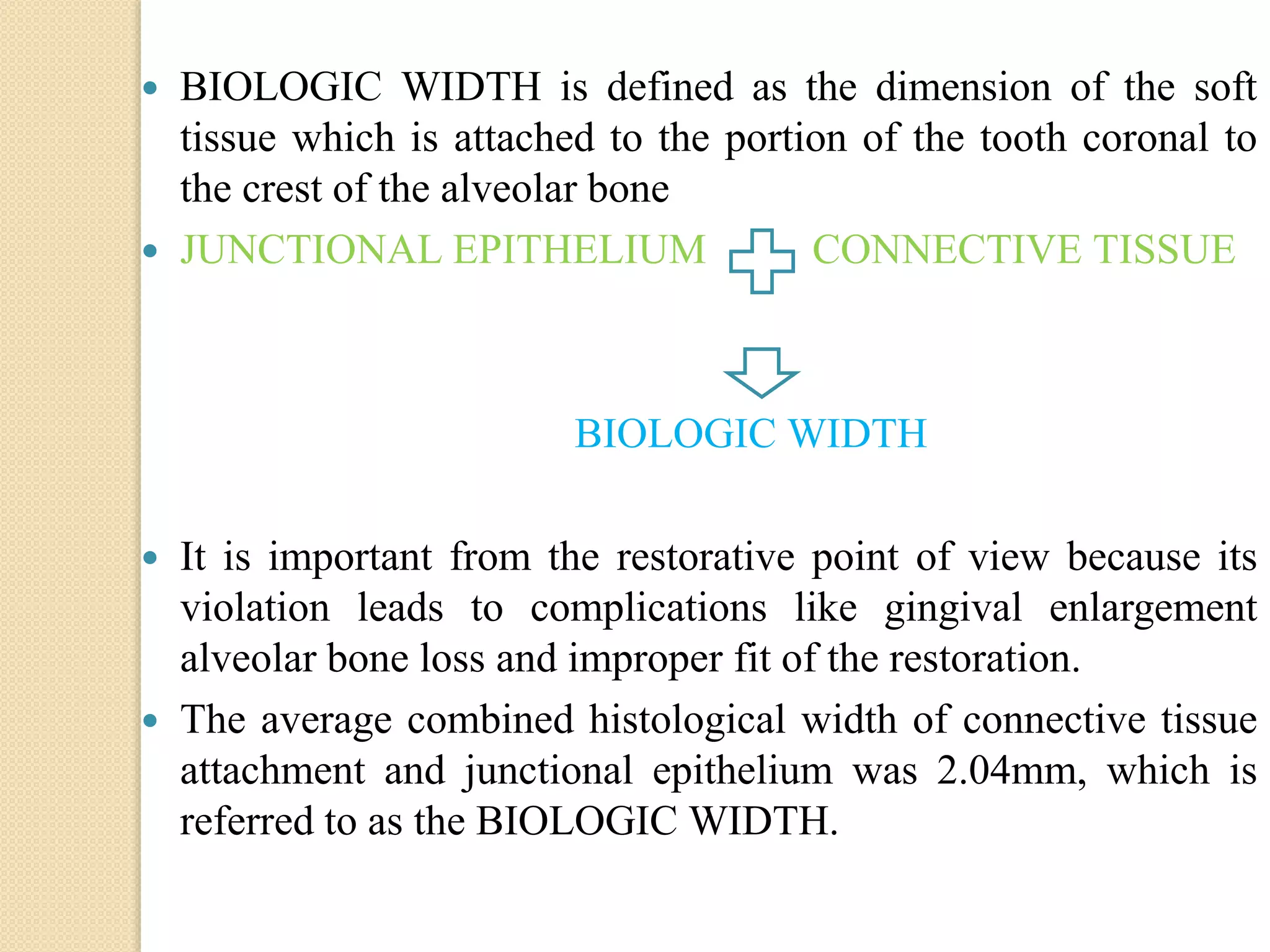
The document discusses the dento-gingival unit, emphasizing the junctional epithelium's structure, functions, and its dynamic role in periodontal health. It covers various aspects including the development, cellular composition, and the attachment mechanisms of the junctional epithelium, as well as its interaction with the gingival fibers and the immune response. Additionally, it highlights the importance of the junctional epithelium in maintaining periodontal health and preventing disease progression.