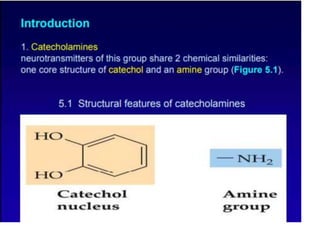
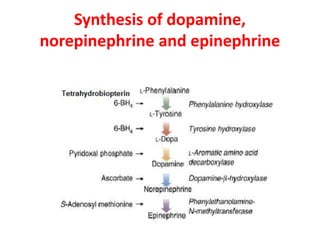
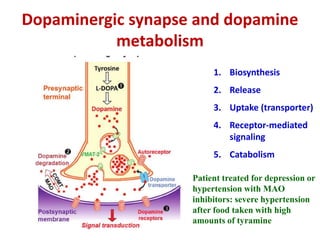
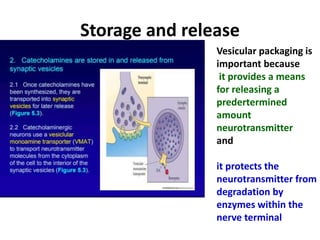
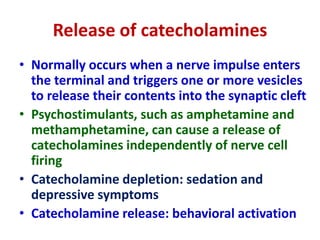
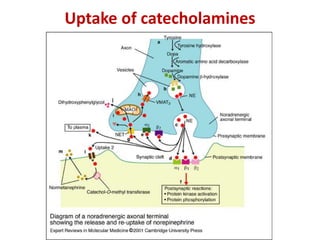
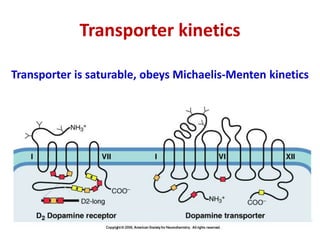
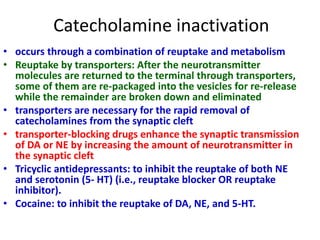
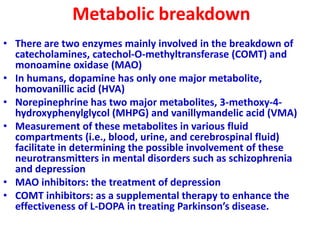
1. The document summarizes the biosynthesis of catecholamines such as epinephrine, norepinephrine, and dopamine.
2. It describes the multi-step enzymatic pathway beginning with tyrosine and involving rate-limiting enzymes such as tyrosine hydroxylase.
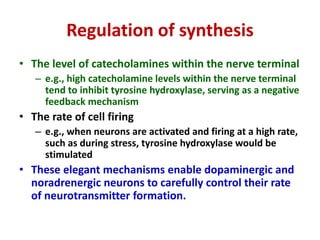
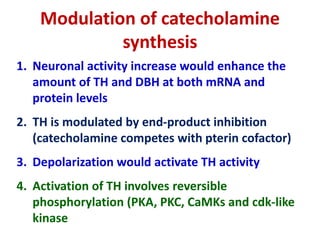
3. Regulation of catecholamine synthesis involves negative feedback and neuronal firing rate control of the enzymes in the pathway.