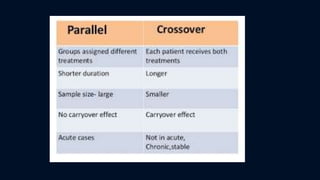
The document discusses the importance of biostatistics and research methodology in scientific research, outlining various types of research including basic, applied, and problem-oriented research. It details experimental designs, particularly emphasizing the need for well-structured experimental designs that include manipulation, control, and randomization. Additionally, it covers the significance of study designs such as observational and experimental studies, including randomized controlled trials and their phases, highlighting their roles in evaluating new treatments and methodologies.