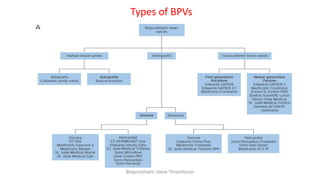
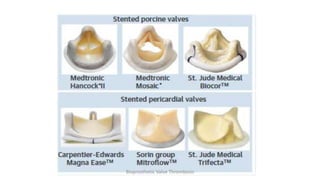
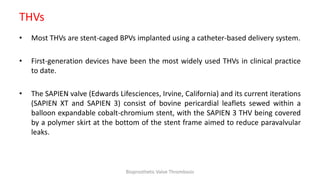
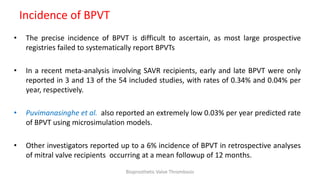
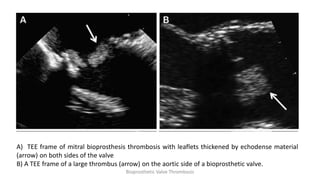
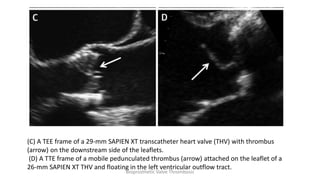
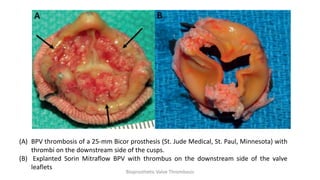
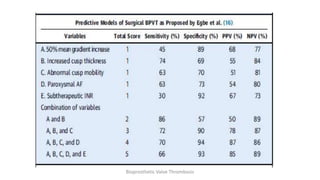
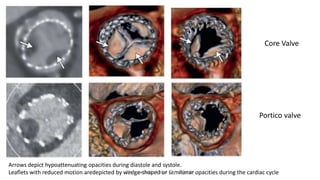
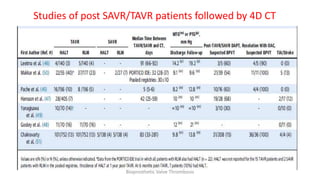
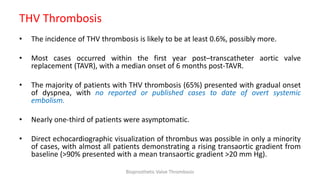
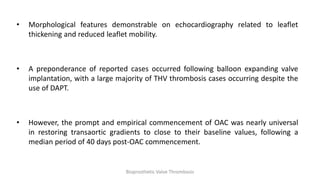
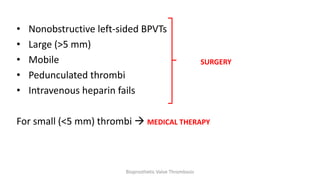
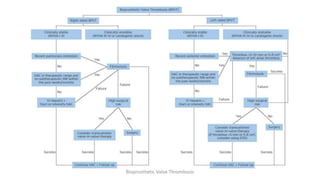
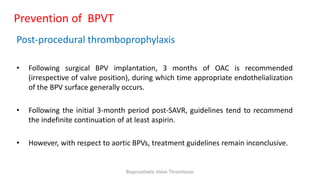
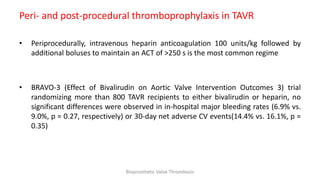
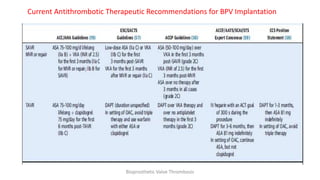
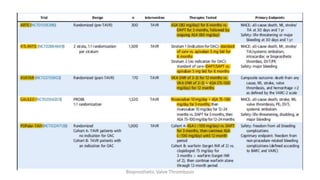
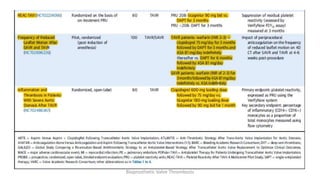
Bioprosthetic valve thrombosis is more common than previously thought and can occur on both surgically and transcatheter implanted valves. While its true incidence is difficult to determine, risk factors include atrial fibrillation, subtherapeutic anticoagulation, obesity, and diabetes. Clinically, it most often presents as worsening dyspnea but can also cause thromboembolism or cardiogenic shock. Echocardiography is key to diagnosis but features like increased gradients, thickened leaflets, and reduced mobility must be considered in the context of the patient's history and risk factors. Treatment involves resumption of effective anticoagulation.