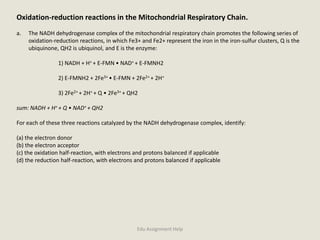
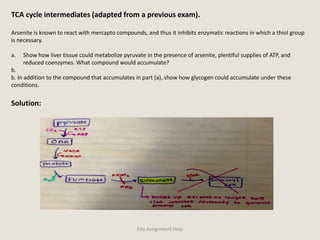
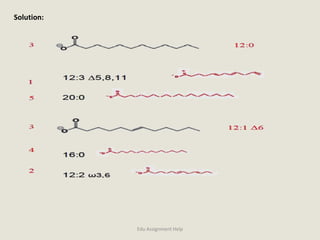
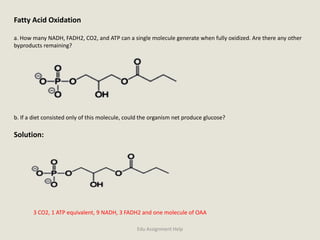
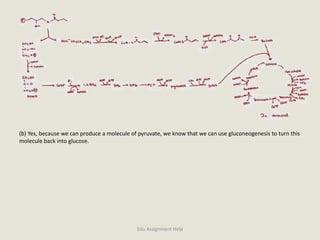
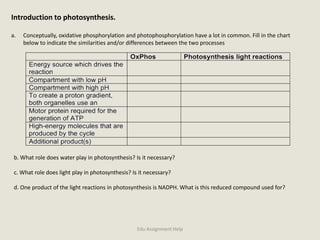
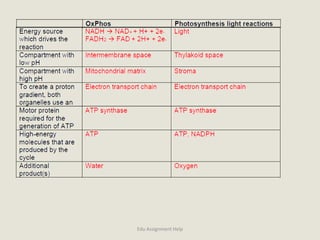
The document provides a detailed analysis of oxidation-reduction reactions occurring in the mitochondrial respiratory chain, emphasizing the role of NADH and FAD as electron carriers. It discusses the conditions affecting the oxidation states of carriers and the effects of inhibitors like potassium cyanide. Additionally, it covers biochemical processes related to fatty acid oxidation and photosynthesis, including the engineering of plants to emit infrared light and the implications on their metabolic functions.