Chapter 2 discusses the properties of water as a solvent in biochemical reactions, including the nature of chemical bonds like polarity, electronegativity, and hydrogen bonding. It contains multiple-choice questions exploring concepts like ionic compounds, micelles, hydrophilic and hydrophobic molecules, and the behavior of acids and bases in solution. Key properties and interactions are highlighted, emphasizing their roles in biological systems.






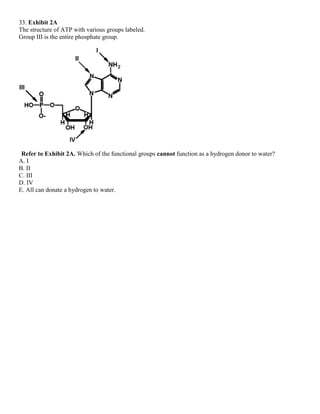
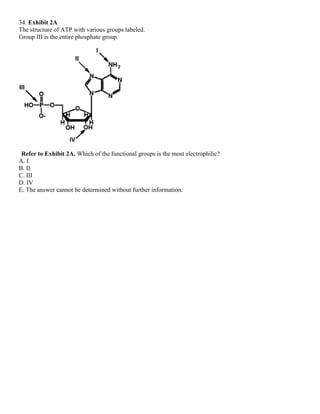
![35. Exhibit 2A
The structure of ATP with various groups labeled.
Group III is the entire phosphate group.
Refer to Exhibit 2A. Which of the groups could not act as a proton acceptor in a hydrogen bond?
A. I
B. II
C. III
D. IV
E. All can accept a hydrogen in a hydrogen bond.
36. Is water an acid or a base?
A. Water is an acid.
B. Water is a base.
C. Water is both an acid and a base.
D. Water is neither an acid nor a base.
37. For an acid that undergoes this reaction:
HA « H+
+ A-
Ka =
A. [H+
][A-
]/[HA]
B. [H+
][HA]/[A-
]
C. [HA][A-
]/[H+
]
D. [A-
]/[HA][H+
]
E. [H+
]/[HA][A-
]](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-9-320.jpg)
![38. Which will dissociate most in water, a weak acid or a strong acid?
A. A weak acid.
B. A strong acid
C. They should dissociate about the same.
D. It's impossible to predict.
39. Bases are
A. proton donors.
B. proton acceptors.
C. hydrogen bond donors.
D. hydrogen bond acceptors.
40. Which has the greater Ka, a weak acid or a strong acid?
A. A weak acid.
B. A strong acid
C. They should dissociate about the same.
D. It's impossible to predict.
41. Which has the greater pKa, a weak acid or a strong acid?
A. A weak acid.
B. A strong acid
C. They should dissociate about the same.
D. It's impossible to predict.
42. The dissociation constant for an acid with a pKa value of 6.0 is
A. 1 ´ 10-6
B. -1 ´ 106
C. 1 ´ 106
D. -1 ´ 10-6
43. A buffer solution at pH 10 has a ratio of [HA]/[A-
] of 10. What is the pKa of the acid?
A. 8
B. 9
C. 10
D. 11
E. 12](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-10-320.jpg)
![44. The dissociation constant for an acid is 1 ´ 10-6
. What is its pKa?
A. -6
B. 6
C. 0.6
D. -0.6
45. The pH of a solution of 0.04 M HCl is:
A. 4
B. 1.4
C. 0.4
D. 0.04
E. The pH cannot be determined
46. The pOH a solution of 0.04 M HCl is:
A. 1.4
B. 10
C. 12.6
D. 13.6
E. The pOH cannot be determined
47. An HCl solution has a pH = 3. If you dilute 10 mL of the solution to 1000mL, the final pH will be:
A. 1.0
B. 2.0
C. The pH does not change.
D. 4.0
E. 5.0
48. If a solution has a pH = 9.6, the [H+
] is
A. 2.5 ´ 1010
B. 9.6 M
C. 2.5 M
D. 2.5 ´ 10-10
M
E. 9.6 ´ 10-10
M
49. What is the pH of a solution with [H+
] = 10 mM?
A. 10
B. 1
C. 2
D. -2](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-11-320.jpg)
![50. Calculate the final pH of a solution made by the addition of 10 mL of a 0.5 M NaOH solution to 500 mL of
a 0.4 M HA originally at pH = 5.0 (pKa = 5.0) Neglect the volume change.
A. 6.10
B. 5.09
C. 7.00
D. 5.55
51. If a solution has a pH = 6, the [H+
] is
A. 6 M
B. 106
M
C. 10-6
M
D. 0.6 M
52. What is the pH of an acetic acid solution where the concentration of acetic acid is 2 mM and the
concentration of sodium acetate is 20 mM. The pKa of acetic acid is 4.76.
A. 5.76
B. 10.6
C. 12.6
D. 8.8
53. The ion product constant for water (Kw) is equal to:
A. 1014
B. 107
C. 100
D. 10-7
E. 10-14
54. In a titration of a weak acid by a strong base
A. two equivalents of base are always needed to neutralize all the acid present
B. the equivalence point cannot be defined exactly
C. there is a region in which the pH changes slowly
D. the equivalence point depends on the nature of the added base
55. A solution at pH 7 contains a weak acid, HA. The pKa of the acid is 6.5. What is the ratio of [A-
]:[HA]?
A. 1:3
B. 1:1
C. 3:1
D. 10:1](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-12-320.jpg)













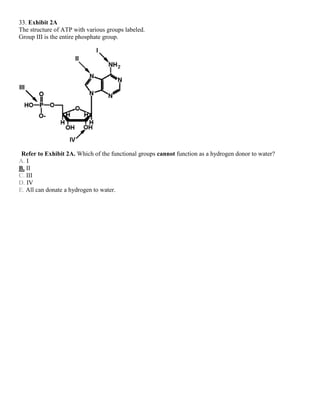
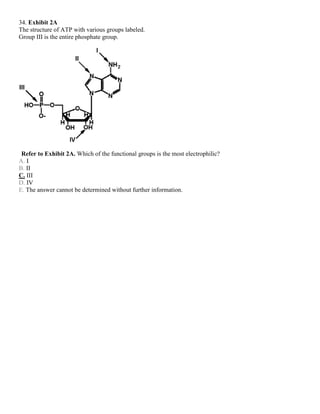
![35. Exhibit 2A
The structure of ATP with various groups labeled.
Group III is the entire phosphate group.
Refer to Exhibit 2A. Which of the groups could not act as a proton acceptor in a hydrogen bond?
A. I
B. II
C. III
D. IV
E. All can accept a hydrogen in a hydrogen bond.
36. Is water an acid or a base?
A. Water is an acid.
B. Water is a base.
C. Water is both an acid and a base.
D. Water is neither an acid nor a base.
37. For an acid that undergoes this reaction:
HA « H+
+ A-
Ka =
A. [H+
][A-
]/[HA]
B. [H+
][HA]/[A-
]
C. [HA][A-
]/[H+
]
D. [A-
]/[HA][H+
]
E. [H+
]/[HA][A-
]](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-28-320.jpg)
![38. Which will dissociate most in water, a weak acid or a strong acid?
A. A weak acid.
B. A strong acid
C. They should dissociate about the same.
D. It's impossible to predict.
39. Bases are
A. proton donors.
B. proton acceptors.
C. hydrogen bond donors.
D. hydrogen bond acceptors.
40. Which has the greater Ka, a weak acid or a strong acid?
A. A weak acid.
B. A strong acid
C. They should dissociate about the same.
D. It's impossible to predict.
41. Which has the greater pKa, a weak acid or a strong acid?
A. A weak acid.
B. A strong acid
C. They should dissociate about the same.
D. It's impossible to predict.
42. The dissociation constant for an acid with a pKa value of 6.0 is
A. 1 ´ 10-6
B. -1 ´ 106
C. 1 ´ 106
D. -1 ´ 10-6
43. A buffer solution at pH 10 has a ratio of [HA]/[A-
] of 10. What is the pKa of the acid?
A. 8
B. 9
C. 10
D. 11
E. 12](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-29-320.jpg)
![44. The dissociation constant for an acid is 1 ´ 10-6
. What is its pKa?
A. -6
B. 6
C. 0.6
D. -0.6
45. The pH of a solution of 0.04 M HCl is:
A. 4
B. 1.4
C. 0.4
D. 0.04
E. The pH cannot be determined
46. The pOH a solution of 0.04 M HCl is:
A. 1.4
B. 10
C. 12.6
D. 13.6
E. The pOH cannot be determined
47. An HCl solution has a pH = 3. If you dilute 10 mL of the solution to 1000mL, the final pH will be:
A. 1.0
B. 2.0
C. The pH does not change.
D. 4.0
E. 5.0
48. If a solution has a pH = 9.6, the [H+
] is
A. 2.5 ´ 1010
B. 9.6 M
C. 2.5 M
D. 2.5 ´ 10-10
M
E. 9.6 ´ 10-10
M
49. What is the pH of a solution with [H+
] = 10 mM?
A. 10
B. 1
C. 2
D. -2](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-30-320.jpg)
![50. Calculate the final pH of a solution made by the addition of 10 mL of a 0.5 M NaOH solution to 500 mL of
a 0.4 M HA originally at pH = 5.0 (pKa = 5.0) Neglect the volume change.
A. 6.10
B. 5.09
C. 7.00
D. 5.55
51. If a solution has a pH = 6, the [H+
] is
A. 6 M
B. 106
M
C. 10-6
M
D. 0.6 M
52. What is the pH of an acetic acid solution where the concentration of acetic acid is 2 mM and the
concentration of sodium acetate is 20 mM. The pKa of acetic acid is 4.76.
A. 5.76
B. 10.6
C. 12.6
D. 8.8
53. The ion product constant for water (Kw) is equal to:
A. 1014
B. 107
C. 100
D. 10-7
E. 10-14
54. In a titration of a weak acid by a strong base
A. two equivalents of base are always needed to neutralize all the acid present
B. the equivalence point cannot be defined exactly
C. there is a region in which the pH changes slowly
D. the equivalence point depends on the nature of the added base
55. A solution at pH 7 contains a weak acid, HA. The pKa of the acid is 6.5. What is the ratio of [A-
]:[HA]?
A. 1:3
B. 1:1
C. 3:1
D. 10:1](https://image.slidesharecdn.com/biochemistry-7th-edition-campbell-test-bank-190413032221/85/Biochemistry-7th-Edition-Campbell-Test-Bank-31-320.jpg)






