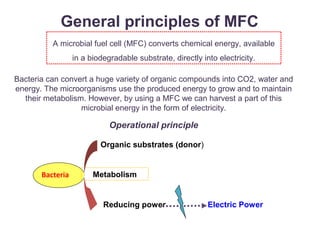
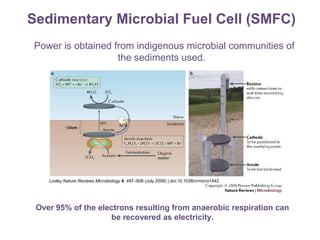
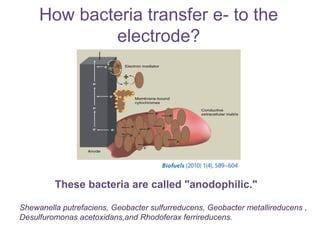
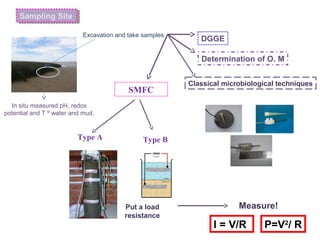
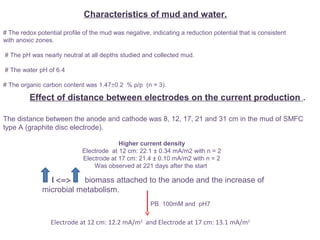
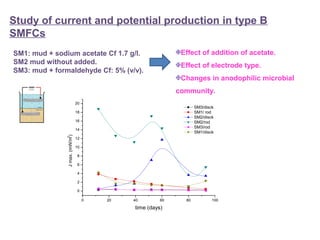
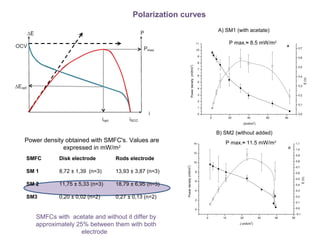
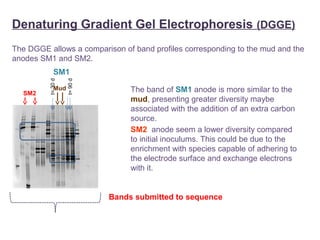
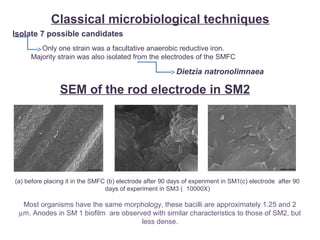
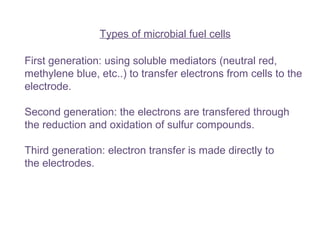
This document summarizes a study that used microbial fuel cells prepared with freshwater sediments from the Rio de la Plata river to produce electricity. The study examined the relationship between current production and changes in the anodophilic microbial community. Microbial communities from the river sediments were able to produce current densities of up to 22.1 mA/m2. Analysis of the anodophilic microbial communities showed that those attached to the anode in fuel cells with added acetate substrate had greater diversity than those without added acetate.