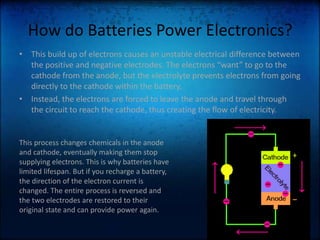
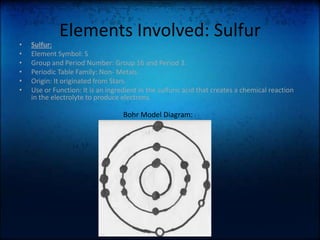
The document discusses how batteries work and the key elements involved. It explains that a battery uses a chemical reaction between its electrodes and electrolyte to produce electrons that flow through an external circuit. The main elements are lithium, sulfur, and zinc. Lithium is used in the anode, sulfur is used in the electrolyte, and zinc can be used in the container. The document also mentions that Toyota is working on developing new magnesium-sulfur batteries for electric cars that could hold twice as much power as lithium-ion batteries.