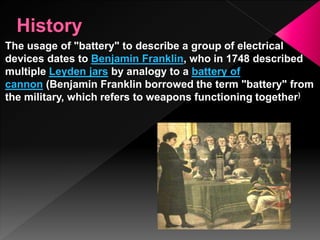
An electric battery is a device that converts chemical energy into electrical energy and consists of one or more electrochemical cells with external connections that provide power to devices. Batteries have a positive terminal called the cathode and a negative terminal called the anode. Primary batteries produce current immediately but cannot be recharged, while secondary batteries must be charged before first use but can be recharged by applying an electric current that reverses the chemical reactions. There are many types of batteries that use different chemical processes and designs to store and release electrical energy.

![An electric battery is a device consisting
of one or more electrochemical cells with
external connections provided to power
electrical devices such
as flashlights, smartphones, and electric
cars.[1] When a battery is
supplying electric power, its positive
terminal is the cathode and its negative
terminal is the anode.](https://image.slidesharecdn.com/batteryelectricity-161227124842/85/Battery-electricity-2-320.jpg)