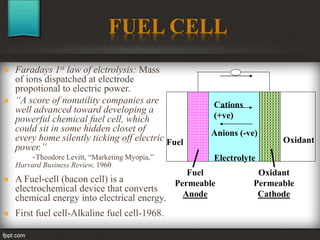
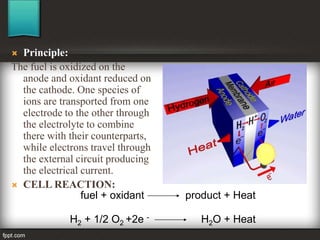
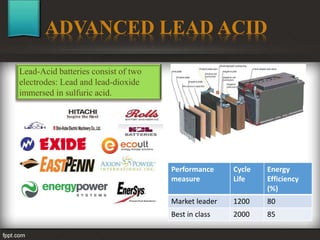
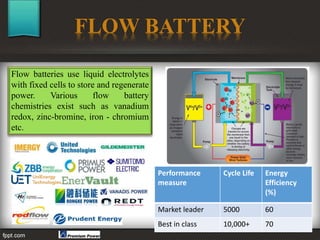
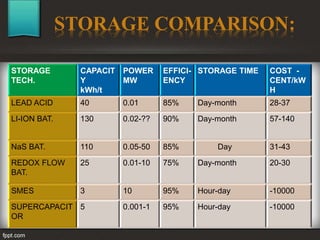
The seminar at Amrutvahini College of Engineering focused on modern electric storage systems and included discussions on various types of storage technologies such as lithium-ion batteries, fuel cells, and super capacitors. Key topics covered included the importance of energy storage, types of fuel cells, advantages and challenges of each storage technology, as well as future trends in energy storage systems. The conclusion emphasized the necessity for reliable and efficient storage systems to avoid energy wastage and respond effectively to peak load demands.