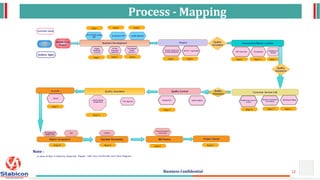
Contoso Ltd. is a GCLP compliant research center located in Bangalore, focused on stability and dosage development for pharmaceuticals, operating since 2010. The company is recognized for its advanced technology integration and quality management supported by a professional team of over 100 scientists, with numerous regulatory approvals including from WHO and the FDA. Their services encompass formulation development, regulatory compliance, stability programs, and microbiological testing, aimed at enhancing product quality in the prevention and cure pharmaceutical segment.