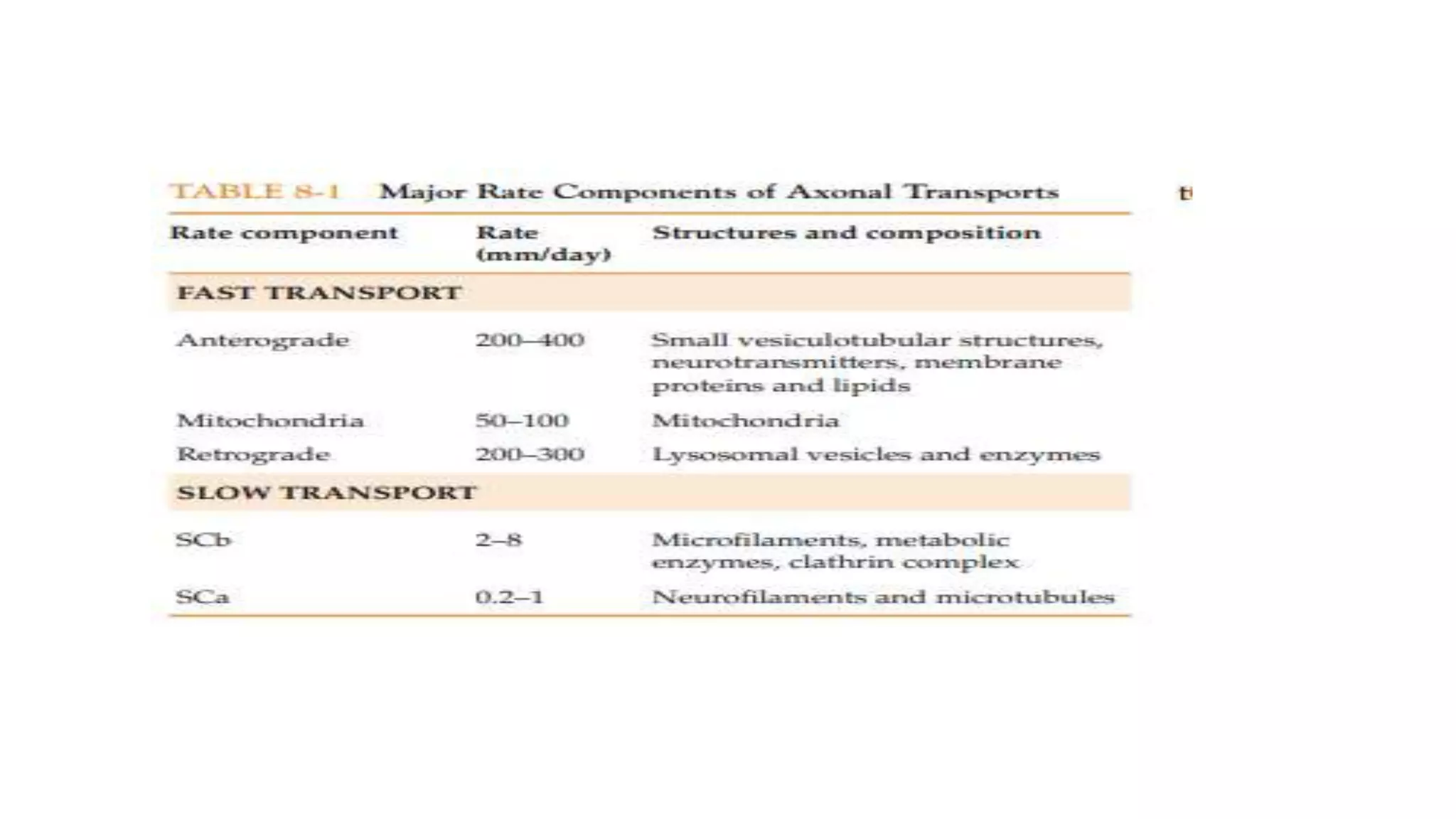
Axonal transport is essential for neuronal survival and function. It involves the bidirectional movement of organelles and molecules along microtubules in axons. Fast axonal transport moves essential components like mitochondria and vesicles down axons at rates of 200-400 mm/day using motor proteins kinesin and dynein. Slow axonal transport moves cytoskeletal elements like neurofilaments and soluble enzymes at slower rates of 1 mm/day, critical for axon growth and regeneration. Defects in axonal transport underlie neurodegeneration in various diseases.