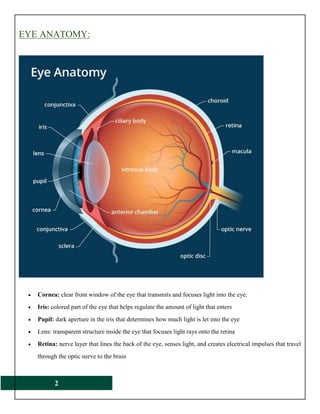
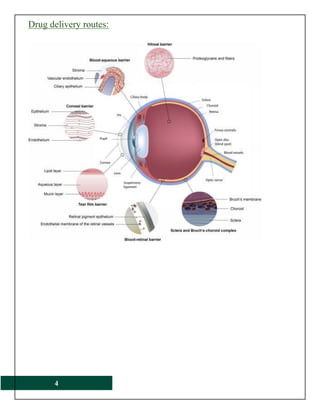
The document provides a comprehensive overview of ophthalmic products, including their definitions, types, and ideal form characteristics for effective ocular drug delivery. It details various drugs used in ophthalmology, administration methods, safety considerations, and formulation components such as preservatives and stabilizers. Additionally, it discusses the anatomy of the eye, bioavailability factors, and critical evaluations for sterility and clarity in ophthalmic products.