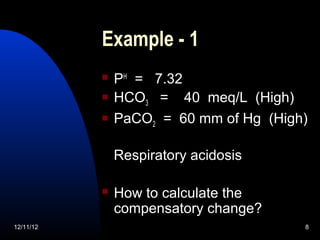
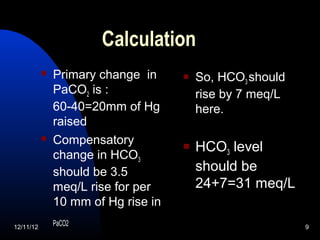
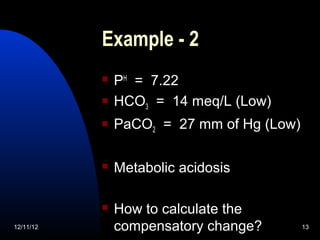
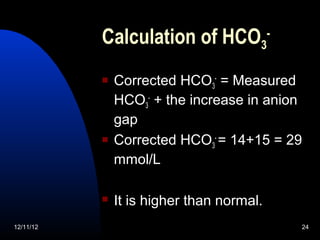
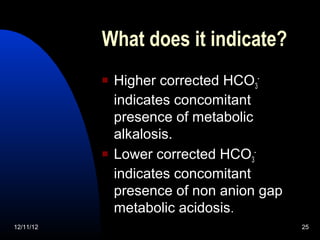
The document outlines a 5-step process for arterial blood gas analysis: 1) Determine if there is acidosis or alkalosis, 2) Calculate compensatory changes to identify mixed disorders, 3) Calculate anion gap, 4) Correct HCO3 levels if anion gap is high, 5) Correlate results with patient's condition. Examples demonstrate using this process to diagnose a patient with respiratory acidosis and metabolic alkalosis in one case, and high anion gap metabolic acidosis with metabolic alkalosis in another.