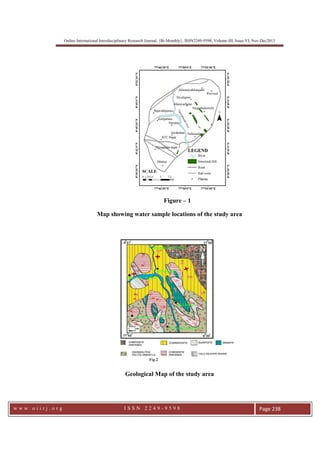
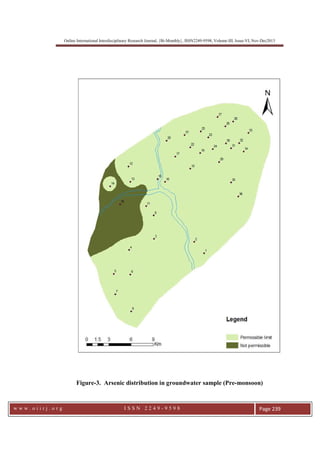
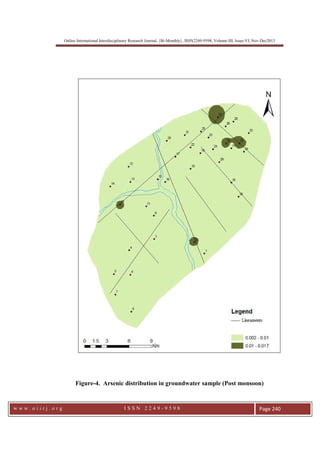
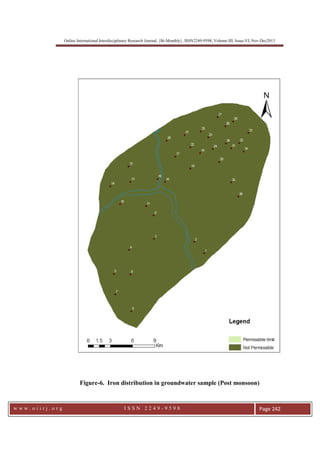
This document summarizes a study of arsenic contamination in groundwater in the Vallanadu region of Tamil Nadu, India. Thirty-six groundwater samples were collected and analyzed for arsenic and other parameters. Arsenic concentrations ranged from 0.003 to 0.017 mg/L, with slightly higher levels found near the Thambraparani River and along geological lineaments. The main sources of arsenic were identified as pesticides used in agriculture and waste from automobiles discharged into the river. Though most samples met standards, concentrations above 0.01 mg/L were found in some locations. The study recommends reducing arsenic-containing pesticides and installing filters to address contamination issues.