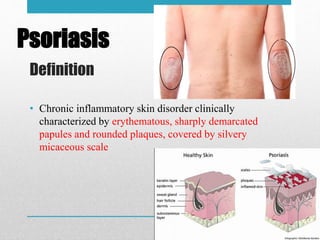
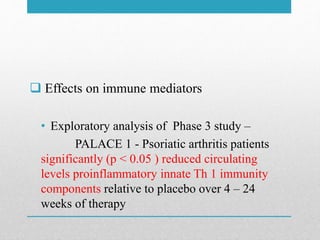
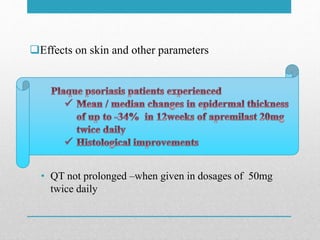
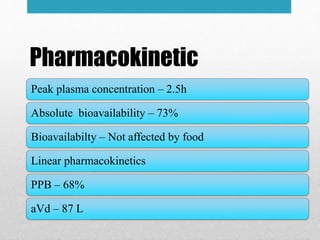
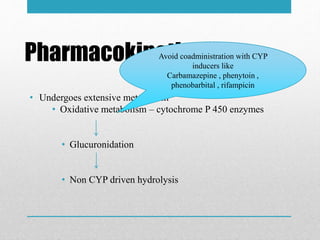
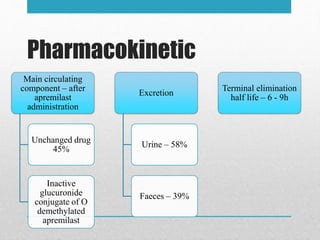
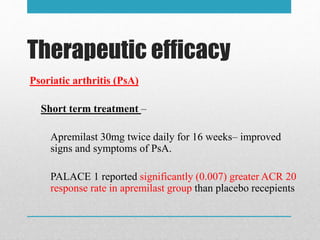
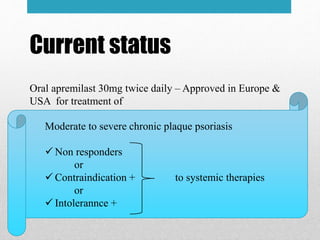
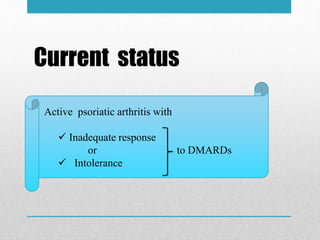
Apremilast is a small molecule inhibitor of phosphodiesterase 4 approved for treatment of moderate to severe plaque psoriasis and active psoriatic arthritis. It works by downregulating inflammatory immune mediators. Pharmacokinetically, it has good oral bioavailability and undergoes extensive metabolism. Clinical trials showed apremilast improved signs and symptoms of psoriasis and psoriatic arthritis over both short and long term use. The most common side effects are diarrhea, upper respiratory tract infection, and nausea. Apremilast is approved in Europe and the US for patients with psoriasis or psoriatic arthritis with inadequate response or intolerance to other systemic therapies.