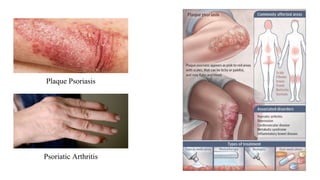
Otezla is a drug created by Celgene Corporation to treat moderate to severe plaque psoriasis and psoriatic arthritis. It works by inhibiting the phosphodiesterase 4 enzyme, which reduces inflammation. Clinical studies showed patients experienced a reduction in symptoms and improvement in their conditions. Common side effects include diarrhea, nausea, and headache. Otezla provides an oral treatment option for these autoimmune disorders.


![Description and Taxonomy:
● Otezla is available orally and is not an injection, cream or biologics.
● Chemically identified as N-[2-[(1S)-1-(3-ethoxy-4-
methoxyphenyl)- 2-(methylsulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-
1H-isoindol-4-yl] acetamide.
● Molecular formula and weight are C22H24N2O7S and 460.5 g/mole,
respectively.
● Apremilast belongs to a class of organic compounds known as
phthalimides. These are aromatic heterocyclic compounds
containing a 1,3-dioxoisoindoline moiety and are imide derivatives
of phthalic anhydrides.](https://image.slidesharecdn.com/otezla-181125132306/85/Otezla-3-320.jpg)

![Clinical Studies :
Randomised, Double
blinded study for evaluation
PALACE-3
(Plaque psoriasis )
PALACE-1
( Psoriatic arthritis
)
PALACE-2
( Psoriatic arthritis)
★ Total of 1493 adult suffering from active psoriatic arthritis participated in studies for evaluation of therapeutic effects of
apremilast.
★ Classification Criteria for Psoriatic Arthritis [CASPAR] with 3 or more swollen joints and 3 or more tender joints was used.
★ Prior to this therapy 76% had non biologic disease modifying antirheumatic drugs (DMARDS) while 22% patients had
biologics DMARDS treatment. Starting dosage of 10 mg was used and later increased to 20 mg or 30 mg for different group.
★ After 5 year prolonged studies promising results were obtained and the condition was assessed using 3 criteria such as
patient pain assessment, physician or physician global assessment etc.
★ Patients responded with ACR 20 response (meaning 20% improvement in overall condition) by week 16. And even ACR 50
or 70 response was recorded with 20 mg or 30 mg dose daily twice.](https://image.slidesharecdn.com/otezla-181125132306/85/Otezla-5-320.jpg)