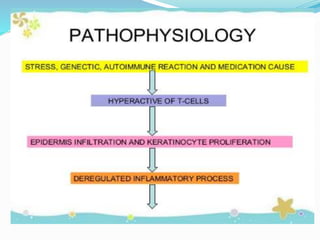
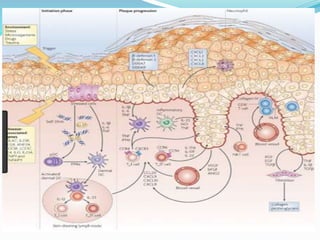
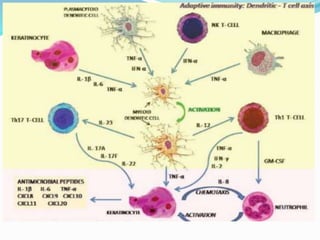
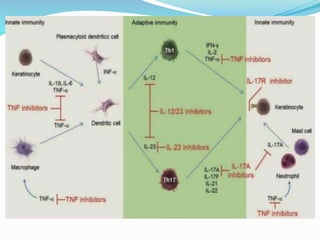
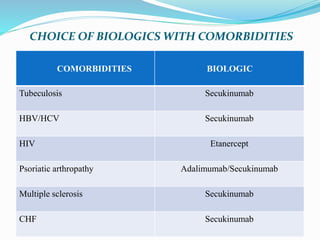
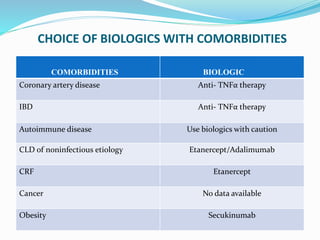
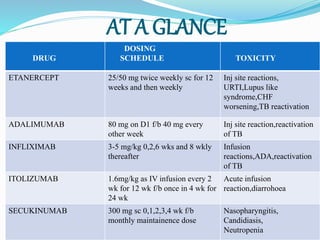
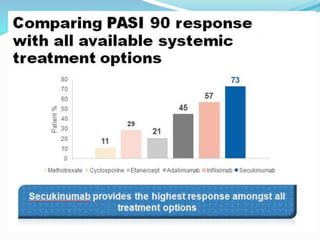
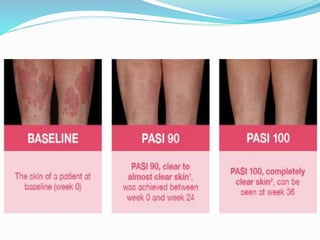
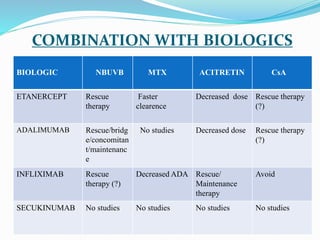
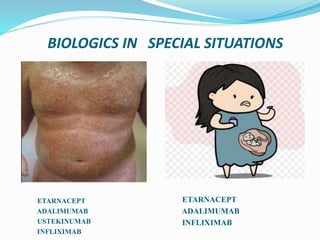
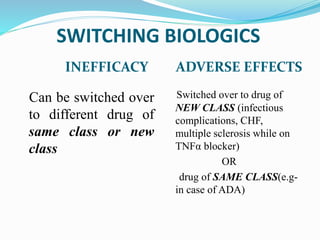
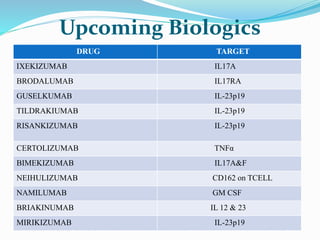
The document outlines the eligibility criteria and considerations for prescribing biologics in psoriasis treatment, including specific comorbidities and recommended biologic agents based on patient profiles. It also highlights the importance of counseling, pre-treatment assessments, and monitoring adverse effects, as well as new biologic therapies on the horizon. Ultimately, secukinumab is noted for its superior efficacy and safety in managing psoriasis.