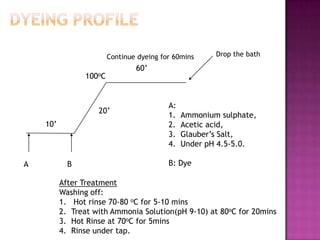
This document summarizes an experiment on dyeing wool with reactive dyes. Wool fiber was dyed using Lanasol reactive dye at 50:1 liquor ratio. It was observed that 96% of the dye was exhausted onto the fiber, leaving 4% remaining in the dye bath. A small amount of dye, approximately 1%, was removed from the fiber during alkaline after-treatment. The dyeing process and after-treatment resulted in even dyeing without tippiness.