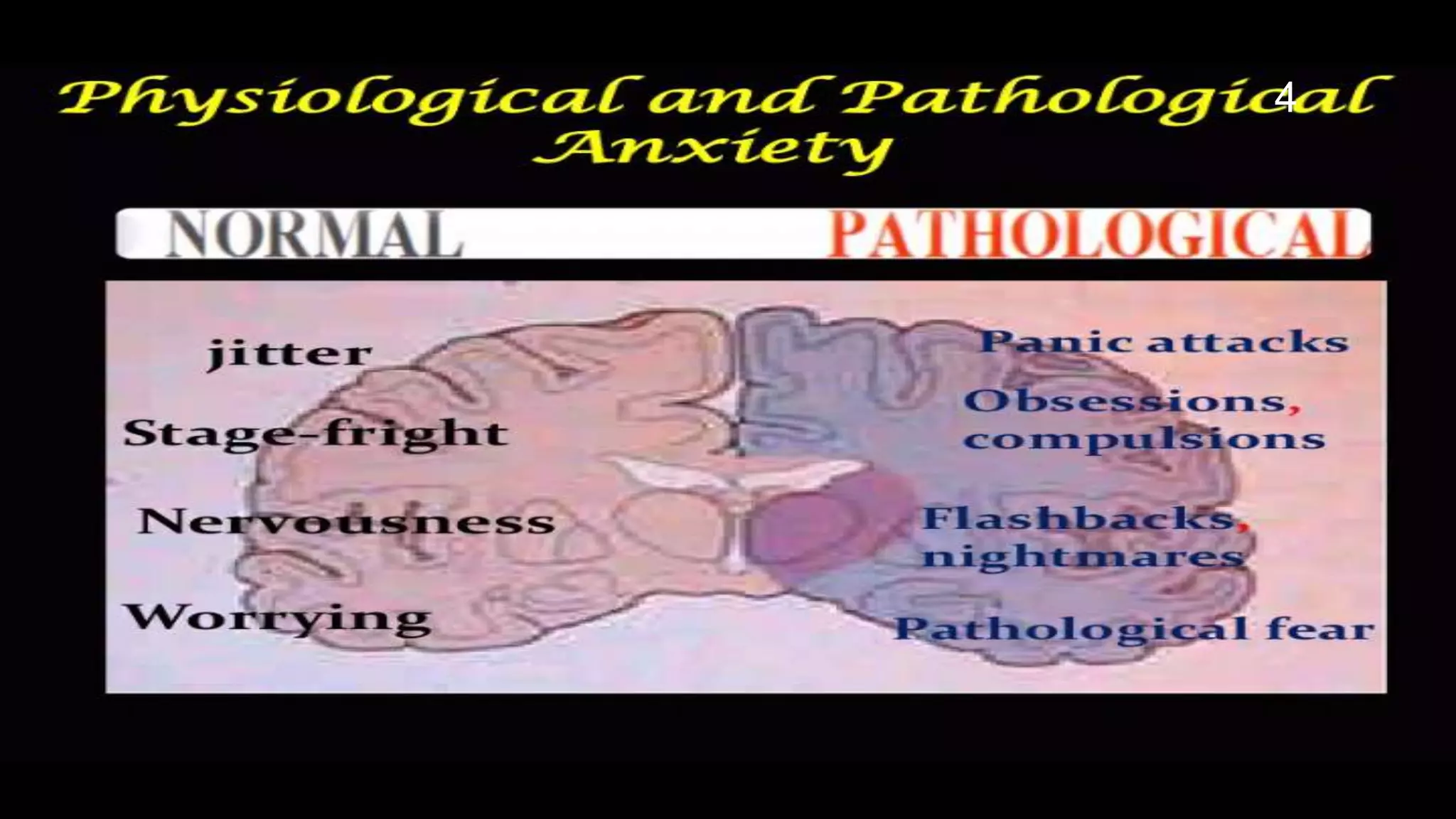
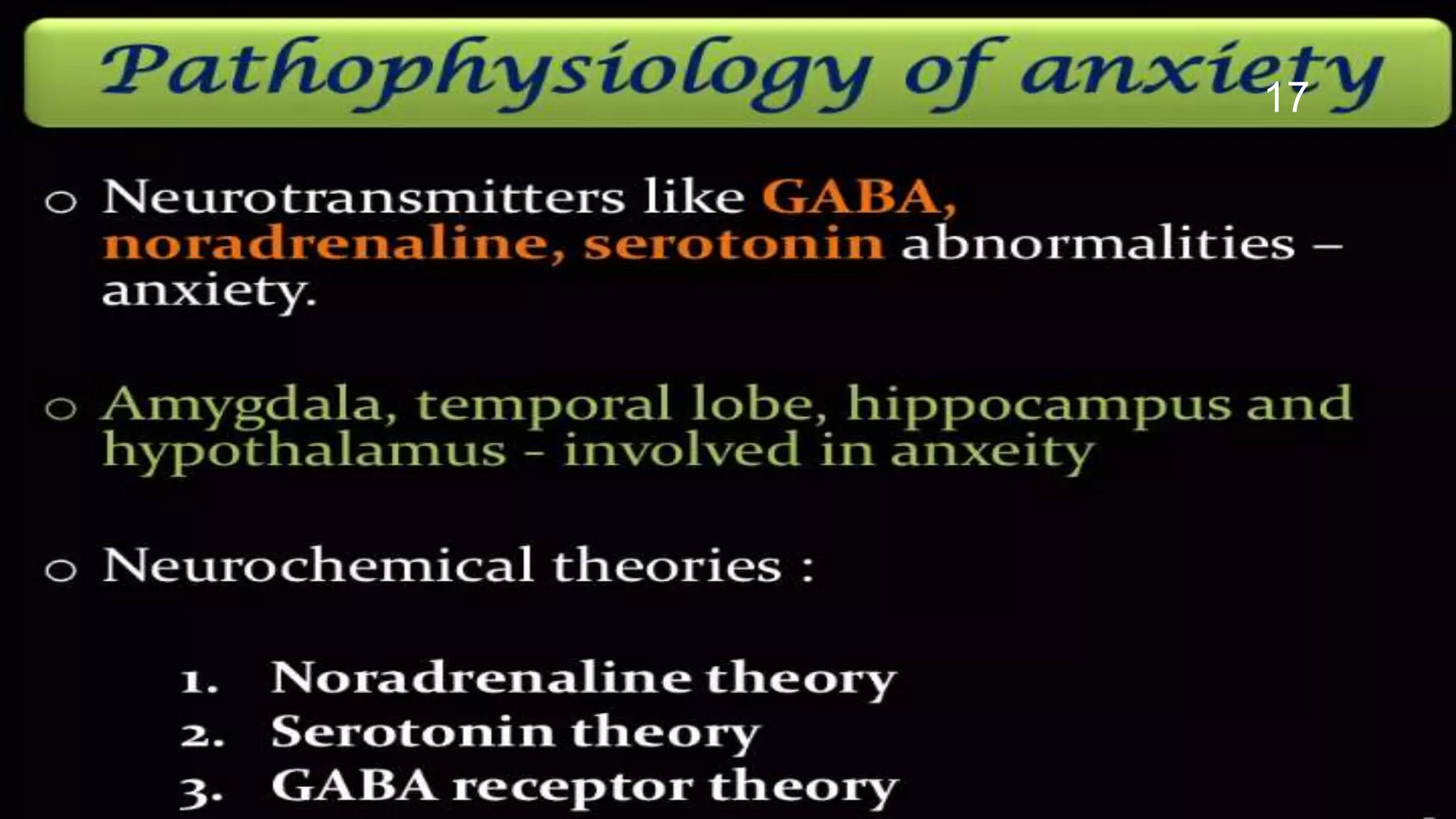
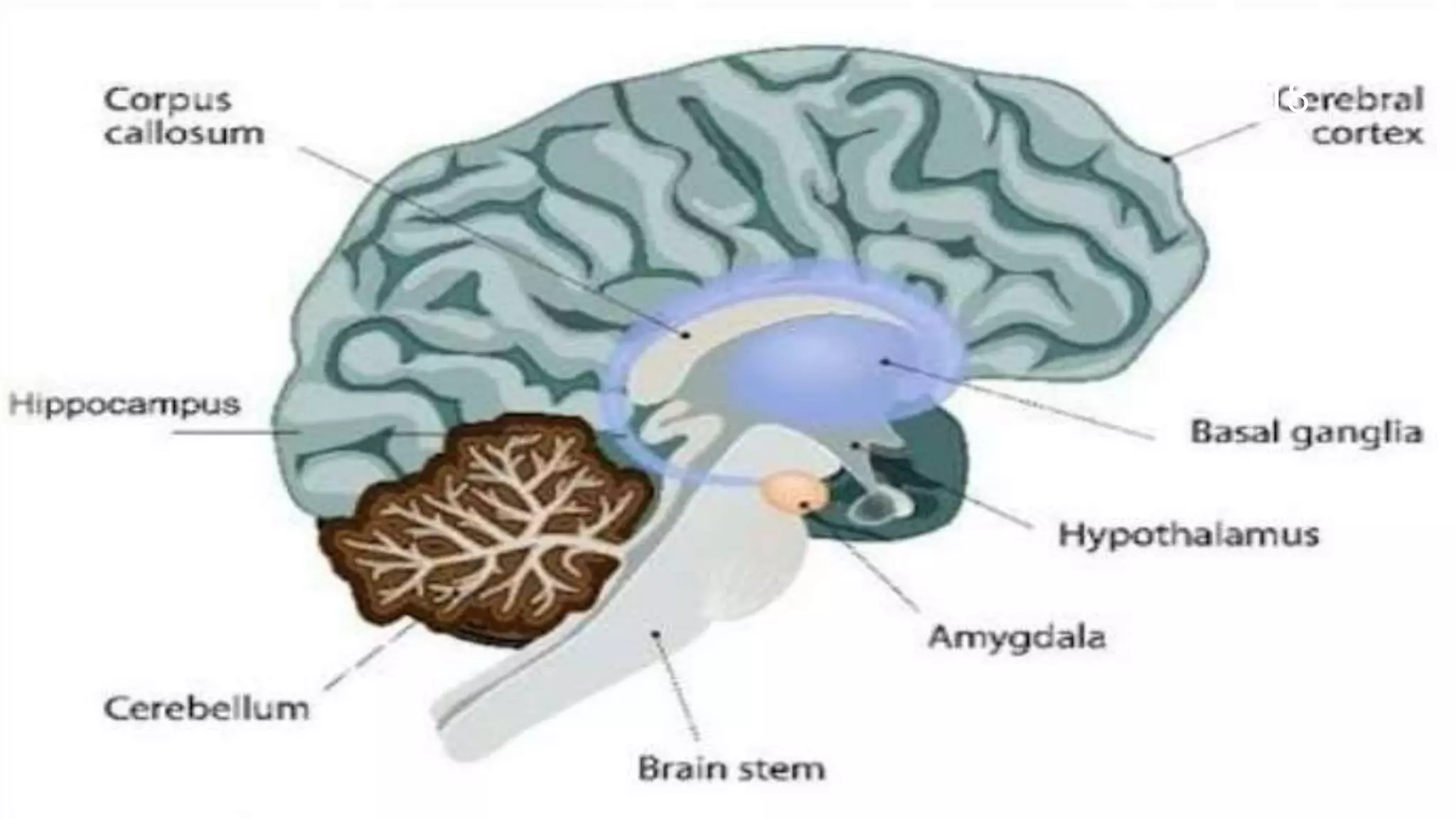
This document describes models used to screen for anxiolytic drugs. It discusses the etiology and types of anxiety disorders like generalized anxiety disorder, panic disorder, phobias, and obsessive compulsive disorder. Symptoms include sweating, dizziness, and muscle tension. Treatments involve psychotherapy, behavior therapy, and medications. Common anxiolytic drug classes are benzodiazepines, azapirones, sedatives, beta blockers, and carbamates. Screening tests measure effects on behavior in animal models and involve GABA receptor binding and effects in tests like the elevated plus maze, social interaction, and water maze tests. The document provides details of the GABA receptor binding assay procedure used to


























![TEST FOR ANXIOLYTIC ACTIVITY
IN VITRO METHODS
GABA Receptor Binding.
• In vitro assay for GABAergic compounds : [ 3H ]-
GABA receptor binding.
• GABAA Receptor Binding.
• GABAB Receptor Binding.
Benzodiazepine Receptor: [ 3H ] – Flunitrazepam
Binding Assay.
30](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-30-2048.jpg)
![ Serotonin Receptor Binding.
• Serotonin ( 5-HT1A ) Receptor: Binding of [ 3H] -8-
Hydroxy- 2-( di-n- Propylamino )- Tetralin ( [3H] - DPAT).
• Serotonin (5- HT1B ) Receptors in Brain : Binding of [3H]
5- Hydroxy tryptamine ( [3H] 5-HT).
• 5- HT3 Receptor in Rat entrohinal cortex Membranes:
Binding of [3H ] GR 65630.
• Histamine H3 Receptor Binding Brain.
31](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-31-2048.jpg)

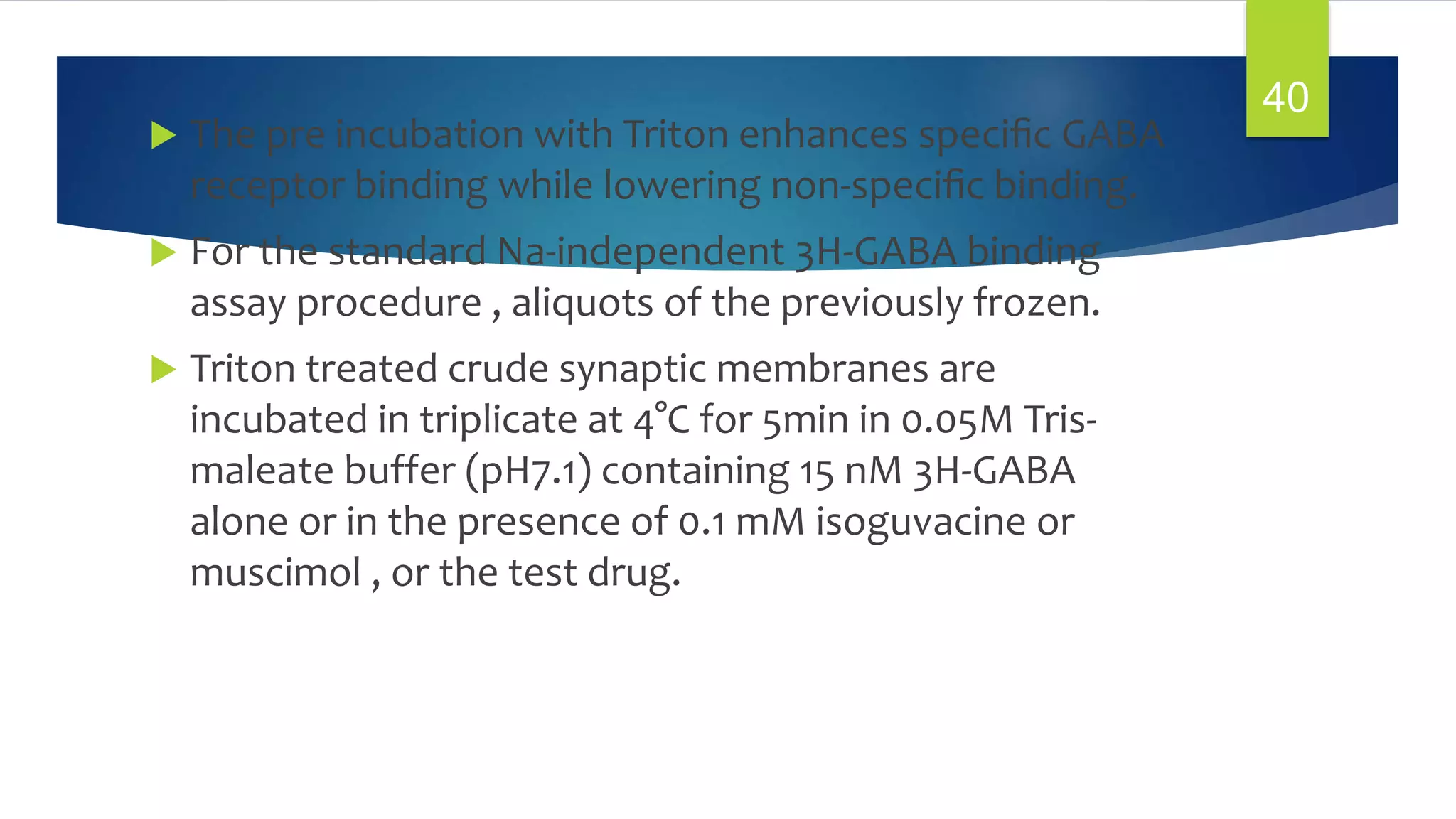
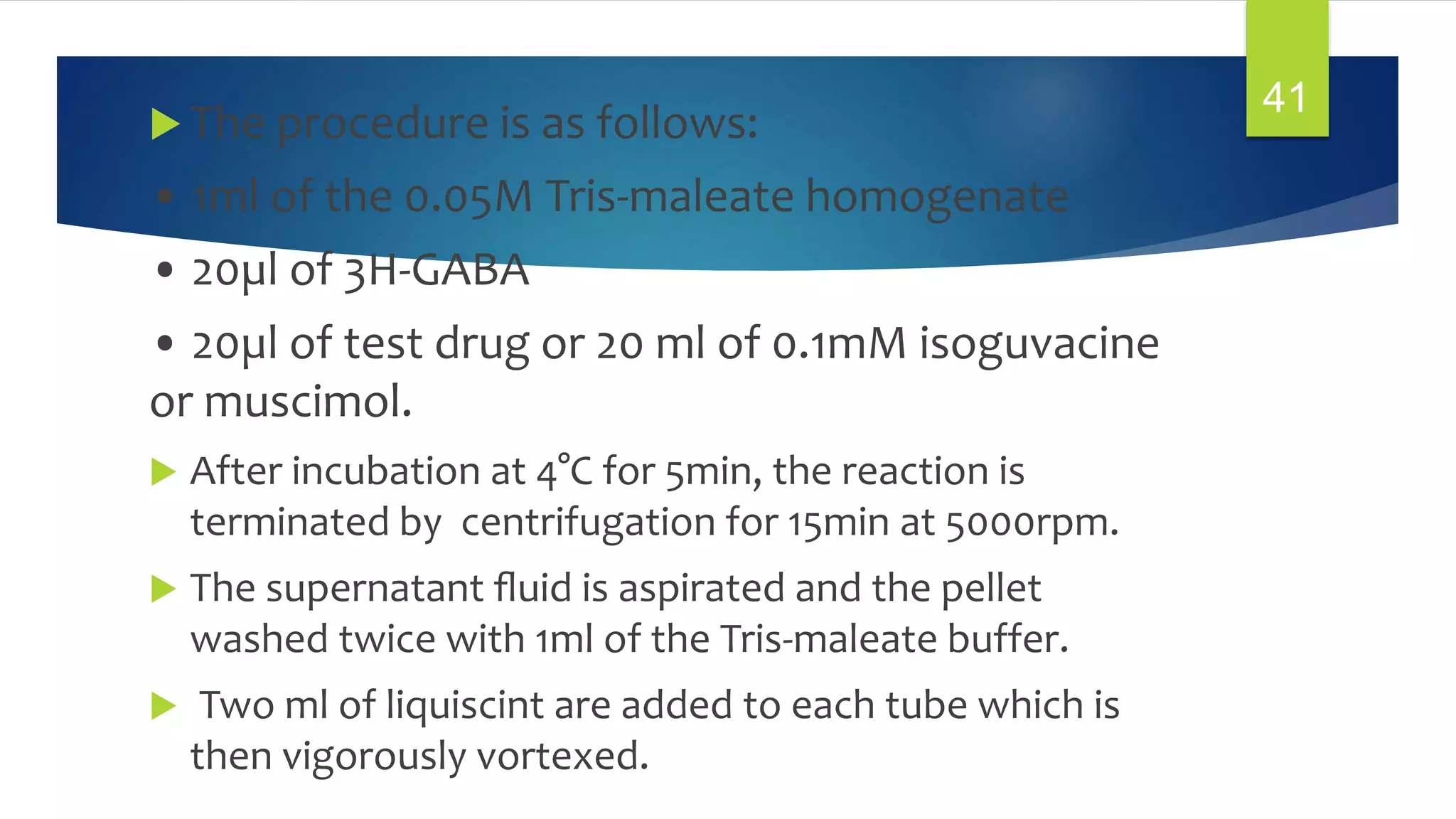
![1. GABA Receptor Binding:
In Vitro Assay for GABAergic Compounds:
[3H]-GABA Receptor Binding:-
PURPOSE AND RATIONALE :
Radiolabeled GABA is bound to synaptic membrane
preparations of mammalian brain. The labeling of the
synaptic receptor with 3H-GABA requires careful
attention to possible interference from non -synaptic
binding since 3H-GABA can also bind nonspecifically to
plasma membranes. The most prominent of which is the
sodium dependent binding of GABA to brain membranes,
a process which appears to be associated with the
transport (uptake) sites of GABA.
33](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-33-2048.jpg)







![2. Benzodiazepine Receptor: [3H]-Flunitrazepam
Binding Assay:
PURPOSEAND RATIONALE
Experiments using 3H-diazepam or 3H-
flunitrazepam have demonstrated specific
binding sites in CNS membrane preparations that
satisfy the criteria for pharmacological receptors.
e.g. saturability, reversibility, stereoselectivity
and significant correlation with in vivo activities of
the drugs in this class.
44](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-44-2048.jpg)


![PROCEDURE
Reagents
• [Methyl-3H]-Flunitrazepam (70–90Ci/mmol ) can be
obtained from New England Nuclear.
• Clonazepam HCl can be obtained from Hoffmann La
Roche.
Tissue Preparation
Male Wistar rats are decapitated and the brains
rapidly removed.
The cerebral cortices are removed, weighed and
homogenized with a Potter-Elvejhem homogenizer in
20 volumes of ice-cold 0.32M sucrose.
47](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-47-2048.jpg)


![3. Serotonin Receptor Binding
Serotonin(5-HT1A)Receptor: Binding
of[3H]-8-Hydroxy2-(di-n-Propylamino)-
Tetralin ([3H]-DPAT)
PURPOSE AND RATIONALE
Determination of the affinity of test
compounds for the 5-HT1A receptor in brain
may be useful for predicting compounds with
novel anxiolytic or atypical anti-psychotic
profiles.
52](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-52-2048.jpg)
![Schlegel and Peroutka (1986) identified [3H]DPAT as a
selective ligand for the 5-HT1A receptor. These authors
reported that [3H]DPAT labeled an autoreceptor. Lesion
studies suggest that [3H]DPAT labeled receptors are not
terminal autoreceptors, but may be somatodendritic
autoreceptors (Gozlan et al. 1983). Although DPAT
decreases the firing rate in the raphe nucleus and inhibits5-
HTrelease,theactuallocationand function is somewhat
controversial (Vergeetal.1986). These studies and the
sensitivity of [3H]DPAT binding to guanine nucleotides and
effects on adenylatecyclase suggest that DPAT acts as an
agonist at the 5-HT1A receptor (Schlegel and Peroutka
1986).
54](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-54-2048.jpg)


![2. [3H]-DPAT (2-(N,N-Di[2,3(n)−3H] propylamino)8-hydroxy-
1,2,3,4-tetrahydronaphthalene) (160–206Ci/mmol ) was obtained
from Amersham. For IC50 determinations: a 10nM stock solution
is made up and 50µl are added to each tube (final
concentration=0.5nM).
3. Serotonin creatinine sulfate. 0.5mM stock solution is made up
in 0.01N HCl and20µl added to 3 tubes for determination of
nonspecific binding (final concentration=10µM).
4.Test compounds:
For most assays, a 1mM stock solution is made up in a suitable
solvent and serially diluted, such that the final concentrations in
the assay range from 2×10−5 to 2×10−8 M. Seven concentrations
are used for each assay. Higher or lower concentrations may be
used based on the potency of the drug.
57](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-57-2048.jpg)

![Assay
800µl Tissue
130µl 0.05M Tris + CaCl2 + pargyline +
20µl vehicle/5-HT/drug
50µl [3H]DPAT
Tubes are incubated for 15min at 25°C.
The assay is stopped by vacuum filtration
Whatman GF/B filters which are then washed 2
with 5ml of icecold0.05M Tris buffer. The filters
placed into scintillation vials with 10ml of
scintillation cocktail and counted.
59](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-59-2048.jpg)
![EVALUATION
Specific binding is defined as the difference between total
binding and binding in the presence of 10µM 5HT. IC50 values
are calculated from the percent specific binding at each drug
concentration. The Ki value may then be calculated by the
Cheng– Prusoff equation:
Ki = IC50/1 + L/KD
The KD value for [3H] DPAT binding was found to be 1.3 nM by
Scatchard analysis in a receptor saturation experiment.
60](https://image.slidesharecdn.com/anxiolyticscreeningmodel-190131055518/75/Anxiolytic-screening-model-60-2048.jpg)