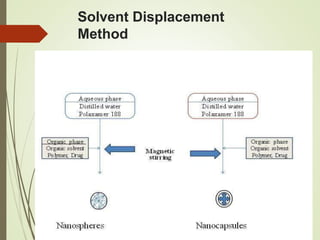
This document discusses nanoparticles for drug delivery. It begins with definitions and concepts, noting that nanoparticles range from 10-1000 nm and can be used to selectively deliver drugs to target sites while reducing effects on non-target tissues. It then covers advantages like reduced dosing and side effects. Methods of preparation include cross-linking and polymerization. Nanoparticles are characterized and evaluated before being discussed for various applications such as cancer therapy, vaccines, and crossing the blood brain barrier.











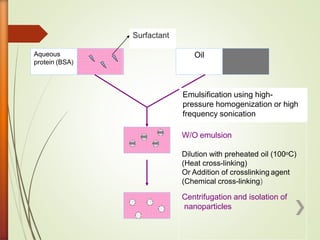




![1) Emulsion polymerization
» It consists of:
» A] Micellar nucleation and polymerization :
Monomer is insoluble in continuous phase.(O/W phase)
Aqueous phase
» B] Homogenous nucleation and polymerization:
Monomer is soluble in continuous phase.(W/O phase)
Organic phase.](https://image.slidesharecdn.com/jaym-200528035930/85/Nanoparticles-17-320.jpg)