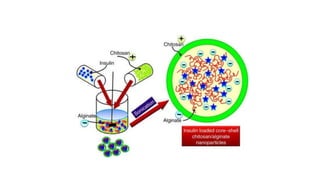
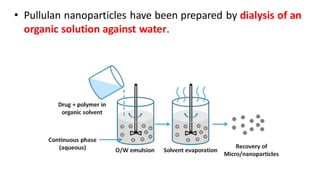
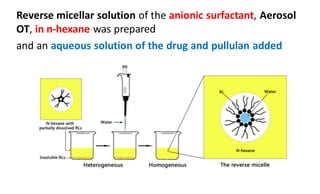
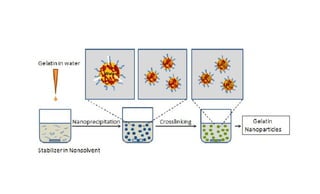
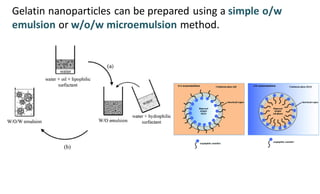
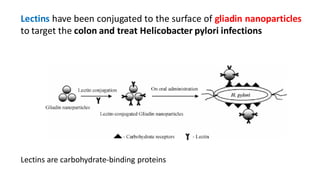
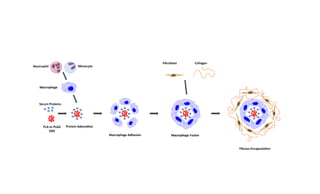
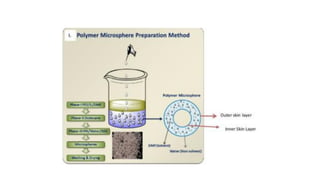
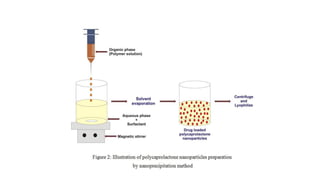
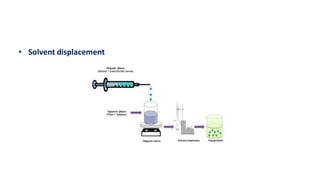
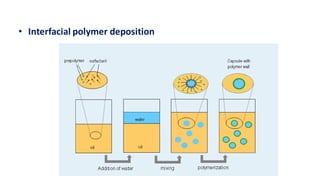
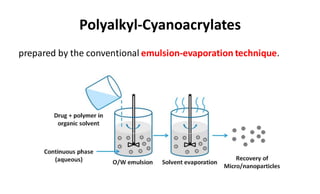
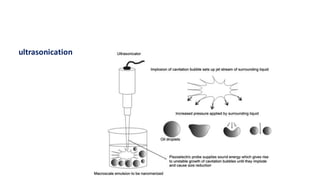
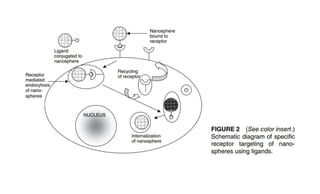
This document summarizes polymers that can be used to prepare nanoparticles for drug delivery. It discusses both natural polymers like alginate, chitosan, pullulan and gelatin as well as synthetic biodegradable polymers like polylactide, polyglycolide and polycaprolactone. Natural polymers may have unpredictable properties but are biocompatible, while synthetic polymers can be precisely designed for controlled drug release and targeting. The document provides examples of how different polymer nanoparticles are prepared and the drugs they can encapsulate and deliver for therapies.